Abstract
Modular organization of proteins has been postulated as a widely used strategy for protein evolution. The multidomain transmembrane protein DsbD catalyzes the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. Most bacterial species do not have DsbD, but instead their genomes encode a much smaller protein, CcdA, which resembles the central hydrophobic domain of DsbD. We used reciprocal heterologous complementation assays between E.coli and Rhodobacter capsulatus to show that, despite their differences in size and structure, DsbD and CcdA are functional homologs. While DsbD transfers reducing potential to periplasmic protein disulfide bond isomerases and to the cytochrome c thioreduction pathway, CcdA appears to be involved only in cytochrome c biogenesis. Our findings strongly suggest that, by the acquisition of additional thiol-redox active domains, DsbD expanded its substrate specificity.
Keywords: CcdA/disulfide bond/DsbD/protein evolution/transmembrane electron transporter
Introduction
Genomes are subjected constantly to genetic events that shape protein evolution. The accumulation of point mutations, insertions and deletions may lead to a gradual divergence of two identical copies of a gene into a system of homologous families that include orthologs and paralogs. A large number of the genes involved in the metabolism of formation and reduction of disulfide bonds in proteins are orthologs and paralogs. They encode variants of a common structural theme, the thioredoxin fold (Martin, 1995), which utilize the same mechanism for transferring electrons from and to their active site Cys-Xa-Xb-Cys motifs.
A single organism may contain >50 members of this family, and these can be located either in the cytoplasm or targeted to compartments such as the bacterial envelope or the lumen of the eukaryotic endoplasmic reticulum. Whereas in the cytoplasm they are part of a complex network of enzymes and small molecules dedicated to the reduction of disulfide bonds (Åslund and Beckwith, 1999), extracytoplasmic members of the thioredoxin superfamily belong to a machinery devoted to the correct and rapid formation of disulfide bonds, a critical step in the folding of nascent proteins (for a review see Ritz and Beckwith, 2001).
Duplications and gene fusion events also contributed to the generation of the immense variety of thioredoxin-like enzymes. This is illustrated by the growing list of eukaryotic protein disulfide bond isomerases, which are characterized by the presence of multiple thioredoxin-like domains (for a review see Ferrari and Söling, 1999). Despite the large body of data on their structure and function, the evolutionary advantage of their modular organization is not clear. More generally, only a limited number of direct experimental approaches have been described to support the fact that the evolutionary fusion of two or more modules resulted in a protein with novel or expanded functions. Here, we provide strong evidence that an evolutionary fusion of a thioredoxin-like and a non-thioredoxin-like thiol-redox active module resulted in the expansion of the substrate specificity of a pre-existing transmembrane enzymatic core, bridging two seemingly unrelated thiol-redox pathways.
The oxidizing environment of the bacterial envelope is established primarily by the powerful oxidant DsbA, which rapidly introduces disulfide bonds in newly synthesized proteins by transferring the electrons to the respiratory chain via DsbB (Bardwell et al., 1991, 1993; Kobayashi et al., 1997). Despite the high oxidizing power of this compartment, certain envelope proteins must be kept reduced. This is the case for bacterial c-type apocytochromes and for periplasmic thiol-disulfide bond isomerases. Bacterial c-type cytochromes are composed of an apopolypeptide chain attached to the vinyl side chain of a heme prosthetic group via thioether linkages (Thöny-Meyer, 2000). A critical step in their maturation pathway is the attachment of the heme cofactor to the polypeptide chain, which occurs after translocation to the periplasm (Thöny-Meyer, 2000). Consequently, the conserved cys teines of the apocytochrome should be maintained reduced as thiolates in order to attack the α-carbons of the vinyl side chains of heme (Monika et al., 1997; Fabianek et al., 1999).
Thiol-disulfide bond isomerases such as DsbC and its homolog DsbG are able to attack disulfide bonds in proteins with multiple cysteines and promote shuffling of non-native disulfide bonds (Zapun et al., 1995; Sone et al., 1997; Bessette et al., 1999). Depending on how the mixed disulfide intermediate between the enzyme and the substrate is resolved, the substrate emerges reduced, with the chance of being re-oxidized by DsbA; or it appears with a rearrangement of its disulfide linkages. If the first reaction takes place, the isomerase exits the reaction with a disulfide bond, and its active site must be reduced before it can participate in further reactions (Walker and Gilbert, 1997; Darby et al., 1998).
A mechanism that supplies electrons to these two periplasmic pathways has been described in Escherichia coli. It involves the cytoplasmic membrane protein DsbD, which utilizes NADPH via the thioredoxin pathway in the cytoplasm as the source of reducing equivalents (Crooke and Cole, 1995; Missiakas et al., 1995; Rietsch et al., 1996, 1997). Accordingly, DsbD can be viewed as a branching point for the electrons coming from the cytoplasm: it reduces DsbC and DsbG (Stewart et al., 1999; Chung et al., 2000), and it also reduces CcmG (Katzen and Beckwith, 2000), which subsequently feeds electrons into the cytochrome c maturation pathway (Fabianek et al., 1999).
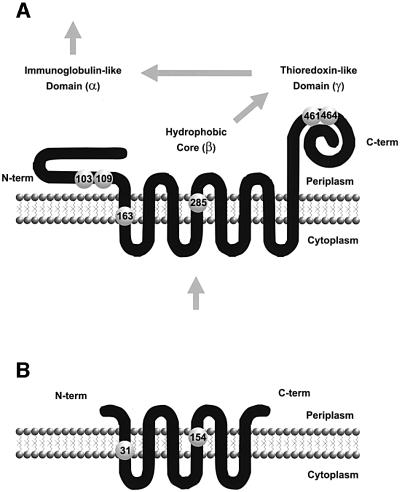
DsbD is organized into three domains: an N-terminal domain (α) with an immunoglobulin (Ig) fold; a central hydrophobic core (β) composed of eight transmembrane (TM) segments; and a C-terminal periplasmic domain (γ) with an apparent thioredoxin-like fold (Stewart et al., 1999; Chung et al., 2000; Gordon et al., 2000; P.W.Haebel, D.Goldstone, F.Katzen, J.Beckwith and P.Metcalf, submitted for publication) (Figure 1A). Each of these three domains includes a pair of cysteines that is essential for the protein’s function (Figure 1A). These domains, when separated and expressed as three independent polypeptide chains, reconstitute an active DsbD. This interesting feature allowed us to determine that electrons are passed directly from cytoplasmic thioredoxin to β, thence to γ, from there to α, and finally to either DsbC or CcmG using a cascade of thiol-disulfide bond reactions (Katzen and Beckwith, 2000) (Figure 1A).
Fig. 1. Membrane topology and structural domains of DsbD and CcdA. Membrane topologies were validated experimentally for E.coli DsbD (A) and R.capsulatus CcdA (B) (see text). Large numbered spheres represent the essential cysteines. The arrows in (A) indicate the direction of the electron flow.
Although DsbD is well conserved among a subset of proteobacteria, a number of bacterial species contain a simpler and smaller homolog, CcdA (Figure 1B). CcdA proteins from Rhodobacter capsulatus and Bacillus subtilis are the best characterized. Both of them were identified in screenings for mutants deficient in c-type cytochromes (Schiött et al., 1997b; Deshmukh et al., 2000). Protein fusion studies paired with cysteine mutant analyses confirmed a six-TM segment model of R.capsulatus CcdA (Figure 1B) and showed that, as in the case of the β domain of DsbD, the invariant cysteines at the end of the first and fourth TM segments are essential for activity (Deshmukh et al., 2000). However, CcdA lacks the corresponding α and γ domains of DsbD. In B.subtilis, ccdA mutants are blocked in a late step of cytochrome c biogenesis and are affected in sporulation (Schiött et al., 1997a; Schiött and Hederstedt, 2000).
The accumulated data suggest that, analogously to DsbD, CcdA might be involved in transmembrane electron transfer. Since CcdA lacks the α and γ domains of DsbD, and the sequence similarity between CcdA and the β domain is marginal (24% identical), the proposal that they are functional homologs requires additional evidence. Here we aimed to address this question by means of heterologous cross-complementation studies using E.coli DsbD and R.capsulatus CcdA as protein models. Our results support the model in which CcdA evolved into the modular DsbD enzyme with broader substrate specificity by the acquisition of thiol-redox active thioredoxin-like and non-thioredoxin-like domains. The expansion of the substrate range permitted the branching of reducing potential coming from the cytoplasm into two independent periplasmic pathways, the disulfide bond isomerization of nascent proteins and cytochrome c biosynthesis.
Results
DsbD and CcdA are functional homologs
The specific step in cytochrome c biogenesis carried out by CcdA has not been ascribed yet. However, it has been suggested that it might be involved in maintaining the apocytochrome c in the correct state for covalent heme attachment (Schiött et al., 1997a). Since the role of DsbD in cytochrome c biosynthesis has already been established (Katzen and Beckwith, 2000), we reasoned that the role of CcdA could be inferred by reciprocal heterologous complementation with E.coli DsbD. As the model CcdA polypeptide, we used the R.capsulatus version.
To study the ability of R.capsulatus CcdA to promote c-type cytochrome biosynthesis in E.coli, a plasmid that expresses this protein fused to the c-Myc epitope was introduced into the E.coli dsbD null strain FED386, and the biosynthesis of the holocytochrome NapB, indicative of DsbD activity (Stewart et al., 1999; Katzen and Beckwith, 2000), was assessed. NapB is a diheme-containing subunit of the periplasmic nitrate reductase (Potter and Cole, 1999). As control experiments, we employed different combinations of the three domains of DsbD; α, β and γ, which were expressed from compatible plasmids as previously described (Katzen and Beckwith, 2000).
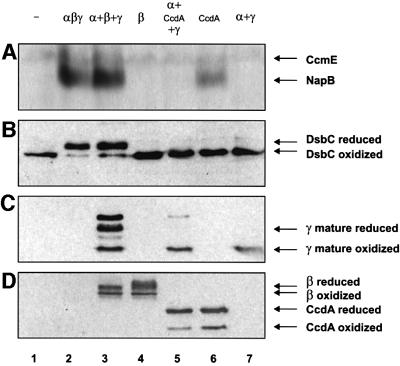
Like E.coli DsbD, R.capsulatus CcdA promoted holo NapB assembly in E.coli (Figure 2A, compare lanes 1, 2, 3 and 6). Although CcdA and the β domain of DsbD were expressed at comparable levels (Figure 2D, lanes 3–6), only CcdA was able to sustain cytochrome c biogenesis in a dsbD null strain on its own; the β domain of DsbD depended entirely on α and γ to complete the electron transfer (Figure 2A, lanes 3, 4 and 6; Katzen and Beckwith, 2000). Surprisingly, when CcdA and the periplasmic domains of DsbD, α and γ, were expressed simultaneously, CcdA was no longer able to sustain cytochrome c biogenesis (Figure 2A, lane 5). Further experiments showed that only α, and not γ, interferes with CcdA activity (data not shown). A possible explanation for this finding is presented below (see roles of α and γ domains of DsbD).
Fig. 2. Phenotypes of E.coli dsbD– cells expressing different combinations of CcdA and DsbD domains. (A) Heme-stained SDS–PAGE of total proteins from stationary phase cells, FED386, induced with 0.2% arabinose and expressing the designated derivatives. The following plasmids were used: pFK093 (lane 2); pFK051, pFK060 and pFK017 (lane 3); pFK060 (lane 4); pFK051, pFK169 and pFK017 (lane 5); and pFK169 (lane 6), pFK051 and pFK017 (lane 7). Lane 1 uses no plasmid. CcmE binds heme through a histidine residue and therefore is not dependent on transmembrane electron transfer. (B) Western blot using antibodies against DsbC. Cells were induced with 0.2% arabinose, harvested at mid-log phase, precipitated with trichloroacetic acid (TCA) and subjected to AMS alkylation. Lanes are the same as in (A). (C) As in (B) but using antibodies against the γ peptide. In lane 3, the first and third bands from top to bottom correspond to reduced and oxidized non-processed forms of γ, respectively. (D) As in (B) but using antibodies against the c-Myc epitope.
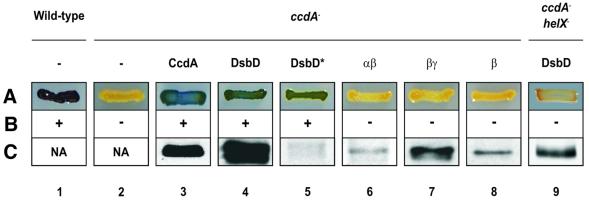
To analyze further the relationships between DsbD and CcdA, we also performed the reciprocal analysis: we investigated the ability of E.coli DsbD to rescue an R.capsulatus ccdA– mutant. The ccdA– strain MD1 is deficient in all known c-type cytochromes (Deshmukh et al., 2000). These electron carrier proteins are essential for various energy transduction pathways in R.capsulatus. For example, cytochrome c1 and either cytochromes c2 or cy are required for photosynthetic growth; and cytochromes co and cp are components of the enzyme cytochrome c oxidase. Accordingly, this mutant is unable to grow photosynthetically and does not produce active cytochrome c oxidase (Deshmukh et al., 2000) (Figure 3, compare lanes 1 and 2).
Fig. 3. Phenotypes of R.capsulatus cells expressing CcdA or different combinations of DsbD domains. (A) Cytochrome c oxidase test. This reflects the capacity of the strains to convert α-naphthol to its oxidized colored form indophenol blue. The relevant genotypes of the strains (MT1131, lane 1; MD10, lane 9; MD1, lanes 2–8) are indicated on top. The following plasmids, expressing the designated genes, were used: pFK225 (lane 3), pFK150 (lane 4), pFK211 (lane 5), pFK154 (lane 6), pFK156 (lane 7), pFK213 (lane 8) and pFK150 (lane 9). Lanes 1 and 2 use no plasmid. In lanes 3, 4 and 6–9, the cloned genes are expressed from the R.capsulatus cycA promoter. In lane 5 (indicated with an asterisk), DsbD is expressed from vector-endogenous promoters. (B) Photosynthetic growth phenotypes. The ability to grow photosynthetically in enriched medium is indicated by symbols (+ or –). Lanes are the same as in (A). (C) Western blot assays using antibodies as follows: anti-c-Myc epitope (lanes 3 and 8), anti-α-peptide (lanes 4, 5, 6 and 9), and anti-γ-peptide (lane 7). Cells in lanes 1 and 2 were not assayed (NA). Lanes are the same as in (A).
Strain MD1 could be readily complemented for these two functions by either CcdA or E.coli DsbD (Figure 3, lanes 1–5). Even very low levels of DsbD, comparable with those in wild-type E.coli, were sufficient to complement the ccdA deficiency (Figure 3, lane 5). However, as is the case in E.coli, the three structural domains of DsbD must be present to restore function. Neither the β domain alone nor the fusions αβ or βγ were sufficient for activity (Figure 3, lanes 6–8).
These results suggest that the three domains of DsbD function as a transmembrane electron transfer unit irrespective of the bacterial host. Conversely, CcdA, which is structurally equivalent to the β domain of DsbD, appears to be sufficient for electron transfer in both R.capsulatus and E.coli.
DsbD and CcdA use a similar set of electron donors and acceptors in the cytochrome c maturation pathway
As part of the apocytochrome c thioreduction pathway, DsbD transfers reducing potential directly from the thioredoxin/thioredoxin reductase system in the cytoplasm to the periplasmic protein CcmG (Katzen and Beckwith, 2000). In the absence of a functional DsbD, CcmG is found completely oxidized (Chung et al., 2000). Since the actual CcdA substrates are unknown, we asked whether CcdA uses the same array of proteins as DsbD to convey electrons to the cytochrome c pathway.
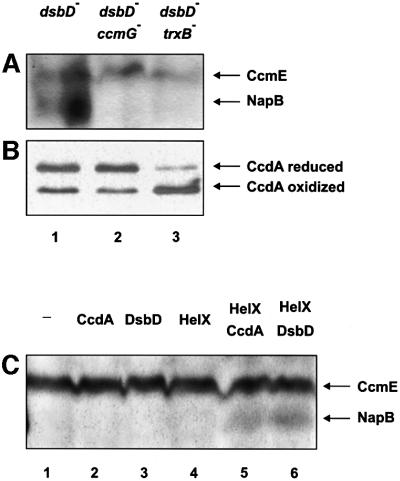
Accordingly, we used the double mutants dsbD–-trxB–, and dsbD–-ccmG– to assess the ability of CcdA to restore cytochrome c biosynthesis in E.coli. Both trxB and ccmG gene products, thioredoxin reductase and CcmG, respectively, are required for DsbD-dependent cytochrome c biosynthesis (Katzen and Beckwith, 2000). A plasmid encoding ccdA was introduced into these strains and the ability to promote biosynthesis of the holocytochrome NapB was studied. As in the case of DsbD, rescue of holo NapB biosynthesis by CcdA in an E.coli dsbD– mutant depends entirely on the expression of CcmG, and on a functional thioredoxin/thioredoxin reductase system (Figure 4A; Katzen and Beckwith, 2000).
Fig. 4. Roles of thioredoxin reductase (TrxB) and CcmG in CcdA activity. (A) Heme-stained SDS–PAGE of total proteins from strains FED386 (lane 1), FED387 (lane 2) and FED762 (lane 3) carrying the plasmid pFK169 and induced with 0.2% arabinose. (B) Western blot showing the in vivo redox state of CcdA. Lanes are the same as in (A). Anti-c-Myc antibodies were used. (C) Heme-stained SDS–PAGE of total proteins from strain FED387 containing the following plasmids: pFK169 (lane 2); pFK093 (lane 3); pFK192 (lane 4); pFK169 and pFK192 (lane 5); and pFK093 and pFK192 (lane 6). Lane 1 uses no plasmid.
To analyze whether the redox state of the two cysteines of CcdA was affected in these two mutant strains, we performed acid trapping of cell extracts followed by 4-acetamido-4′maleimidylstilbene-2,2′-disulfonic acid (AMS) alkylation (see Materials and methods). The high molecular weight alkylating reagent, which reacts specifically with thiolates, allows ready distinction between oxidized and reduced forms of protein dithiols.
In a background where only dsbD is mutated, CcdA is found with its two cysteines reduced (Figure 4B, lane 1). As expected, the redox state of CcdA was not greatly altered by a further deletion of the ccmG gene (Figure 4B, compare lanes 1 and 2). However, in the absence of cytoplasmic reduced thioredoxins, CcdA was mainly in the oxidized form (Figure 4B, lane 3). These results strongly suggest that CcdA acts by transferring electrons from the cytoplasmic thioredoxin/thioredoxin reductase system to periplasmic substrates.
In parallel, we asked whether E.coli DsbD depends on the presence of a similar set of proteins in R.capsulatus. Consistent with an earlier report, which suggested that the thioredoxin/thioredoxin reductase system is essential in the Rhodobacter group (Pasternak et al., 1997), we were unable to knock out the corresponding genes in R.capsulatus; therefore, the role of the cytoplasmic reducing potential in R.capsulatus in the reduction of CcdA or DsbD could not be verified. However, the ccmG homolog helX, which is essential for cytochrome c biosynthesis in R.capsulatus, can be inactivated readily (Beckman and Kranz, 1993). Hence, to study whether HelX is involved in the delivery of the electrons that were transferred from the cytoplasm by DsbD, we generated a double mutant ΔhelX-ΔccdA. A plasmid expressing DsbD was introduced into this strain, and the photosynthetic growth capacity together with cytochrome c oxidase activity was assessed. As was the case in E.coli, we observed that in R.capsulatus DsbD depended on the expression of HelX for complementation of a ccdA deficiency (Figure 3, lane 9).
To confirm further that HelX is truly a CcmG functional homolog, indicating that it operates as electron acceptor from DsbD/CcdA, we asked whether the pairs DsbD–HelX and CcdA–HelX are capable to reconstitute the cytochrome c biogenesis pathway in an E.coli dsbD-ccmG double mutant (FED387). When CcdA, DsbD and HelX were expressed individually in E.coli strain FED387, they failed to rescue cytochrome c biosynthesis (Figure 4C, lanes 1–4). However, when HelX was expressed simultaneously either with DsbD or with CcdA, NapB biosynthesis was partially restored (Figure 4C, lanes 5 and 6).
Taken together, our results suggest that despite the structural differences, DsbD and CcdA catalyze a similar transmembrane reaction in the cytochrome c thioreduction pathway, shuttling electrons from cytoplasmic thioredoxins to the periplasmic CcmG or HelX.
Roles of α and γ domains of DsbD
One of the hallmarks of DsbD is its ability to transfer reducing potential to a number of periplasmic substrates. Besides being involved in the cytochrome c biogenesis pathway, DsbD also maintains the thiol-disulfide bond isomerases DsbC and DsbG in their competent reduced state (Rietsch et al., 1997; Bessette et al., 1999).
Since homologs to these proteins could not be found within the finished genome sequences of R.capsulatus and other members of the Rhodobacter group, we aimed to study in vivo whether R.capsulatus CcdA can serve as an electron donor for E.coli DsbC. We employed the dsbD null strain FED386 containing different components of the transmembrane electron transport system to determine the thiol-redox state of DsbC. While DsbD reduces DsbC, even when it is expressed as three independent polypeptide chains, no reduced DsbC could be detected when CcdA was expressed alone or in combination with the α and γ domains of DsbD (Figure 2B).
Our results have two implications. First, it appears that DsbD has broader substrate specificity than CcdA. Secondly, our data also suggest that, in contrast to the β domain of DsbD, CcdA cannot channel electrons to the periplasmic domains of DsbD; otherwise the concomitant expression of CcdA, α and γ would have restored DsbC reduction (Figure 2B).
To validate this last hypothesis, we determined the thiol-redox state of the γ domain, in the presence of either CcdA or β. As expected, while the central hydrophobic core domain of DsbD was capable of partially reducing γ (Figure 2C, lane 3; Katzen and Beckwith, 2000), the redox state of mature γ remained fully oxidized when CcdA, rather than β, was included in the system (Figure 2C, compare lanes 5 and 7).
The inability of CcdA to sustain electron flow through γ and α allows a plausible explanation for why these accessory domains block the cytochrome c thioreduction pathway in a dsbD– strain expressing CcdA (Figure 2A, compare lanes 5 and 6). In the absence of an electron source, γ can no longer reduce α, which in turn may behave as an oxidant rather than a reductant, promoting the oxidation of CcmG. Consequently, when CcdA and the DsbD domains α and γ are co-expressed, CcmG will participate in two opposing reactions: (i) the forward reaction, in which CcdA reduces CcmG; and (ii) the reverse reaction, where oxidized α drains reducing equivalents back from CcmG. Alternatively, oxidized α might interact with CcmG, sterically inhibiting the electron transfer from CcdA. As a result, the electron pathway is disrupted and the apocytochromes can no longer be kept reduced.
Distribution of CcdA, DsbD and DsbC among living organisms
We have shown that, while both proteins, DsbD and CcdA, can operate equally well in the cytochrome c biogenesis pathways of E.coli and R.capsulatus, only DsbD is capable of reducing DsbC. This broader substrate specificity of DsbD appears to be due to the presence of the Ig-like periplasmic domain α. Therefore, the sequence of α can be used as a tool to distinguish between DsbD-like and CcdA-like proteins.
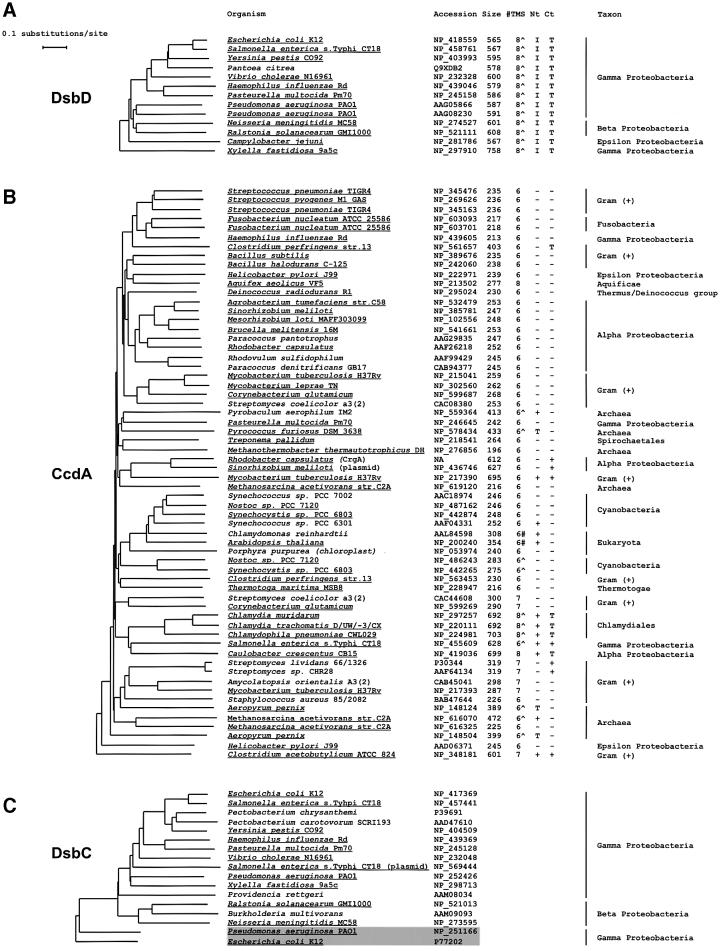
A search for DsbD homologs, employing the sequence of the α domain of E.coli DsbD as a query sequence, revealed that DsbD-like proteins, all of them containing α, β and γ domains, are restricted to β and γ proteobacteria, with the exception of the ε proteobacterium Campylo bacter jejuni, which also contains a DsbD (Figure 5A). In contrast, homologs to CcdA, which lack the Ig-like domain α, are widespread (Figure 5B). They are found not only in genomes throughout the whole eubacteria kingdom, but also in archaea and in plant genomes. Altogether, these data suggest that the origin of CcdA pre-dates the divergence of DsbD. This supposition is consistent with the facts that: (i) in many cases, a gene encoding a thioredoxin-like protein with a signal sequence is located adjacent to ccdA (Page et al., 1997); and (ii) some CcdA homologs enclose a thioredoxin-like domain either at their N- or C-termini (Figure 5B). These examples may reflect an earlier stage in the evolution of DsbD.
Fig. 5. Phylograms of DsbD, CcdA and DsbC family members. Trees were generated by comparing the full-length sequences of (A) DsbD, (B) CcdA and (C) DsbC, as indicated in Materials and methods. DDBJ/EMBL/GenBank accession numbers are shown next to the name of the organism. For the sequences in (A) and (B), the number of residues (Size), and the number of predicted TM segments (TMS) are shown. The presence of a putative signal sequence ‘∧’ in addition to the TM segments, or a putative chloroplast transit peptide ‘#’ is indicated. Extended N- (Nt) and C- (Ct) terminal domains, in addition to the hydrophobic core, are cataloged as thioredoxin- (T), Ig- (I) or other folds (+). Underlined text represents data corresponding to finished genome projects. Only one set of data was taken into account for those organisms where more than one completed genome is available. All the DsbD- and CcdA-like sequences have invariant cysteine residues in predicted TM segments 1 and 4, with the exception of sequences NP_348181 (C.acetobutylicum ATCC 824)and NP_213502 (A.aeolicus VF5), in which those are included in predicted TM segments 2 and 5, and 1 and 6, respectively. With the exception of X.fastidiosa DsbD, all of the DsbD sequences cluster in a separate branch in the CcdA tree (not shown). The shaded box in (C) groups DsbG sequences. The DDBJ/EMBL/GenBank accession number for R.capsulatus CrgA is not available (NA).
While dsbD-like gene products are very homogeneous in size and in number of predicted TM segments, members of the CcdA family exhibit a more complex similarity profile. Although most of its members are polypeptides smaller than 300 amino acids with six predicted TM segments, some others are longer with an increased number of predicted TM segments and variable N- and C-termini (Figure 5). For example, Salmonella species contain members of the DsbD and CcdA families, where the predicted ccdA-like gene products show extended N- and C-termini with no apparent similarity to Ig and thioredoxin folds. Other genomes, such as that of R.capsulatus, encode two members of the CcdA family: CcdA itself and the homolog CrgA, with six predicted TM segments, which apparently is not involved in cytochrome c biogenesis (M.Deshmukh, M.Valkova-Valchanova and F.Daldal, in preparation).
Although all the known substrates of both DsbD and CcdA are members of the thioredoxin family, only DsbC and its homolog DsbG include an N-terminal dimerization domain, which confers on them a number of unique properties (McCarthy et al., 2000; Sun and Wang, 2000; Bader et al., 2001). The presence or absence of this particular feature allows a ready distinction between periplasmic protein disulfide bond isomerases and other periplasmic thioredoxins. This domain, which is essential in the interaction between DsbC and DsbD (Goldstone et al., 2001), might hinder the interaction between DsbC and CcdA.
Our results on the functional link between DsbD and DsbC lead us to predict that these two proteins would have the same phylogenetic profile. Accordingly, the distribution of the DsbC homologs among living organisms resembles that of DsbD (Figure 5A and C), clustering within the β and γ subdivisions of proteobacteria. With the exception of the ε proteobacterium C.jejuni, all the finished genome sequences that contain dsbD also contain a dsbC-like gene, and vice versa. On the other hand, most of the genomes that encode a CcdA-like protein do not appear to have a dsbC or dsbG gene. In particular, R.capsulatus (α proteobacterium) and B.subtilis (Gram positive), where CcdA has been studied in more detail, do not have DsbC or DsbG homolog sequences.
Discussion
In this study, we used reciprocal heterologous complementation assays between E.coli and R.capsulatus to determine the function of the transmembrane protein CcdA. Our results support the proposal that, like DsbD, CcdA mediates the transfer of reducing potential from the cytoplasm to the periplasm.
The fact that DsbD and CcdA are functional homologs is somewhat surprising as CcdA lacks the periplasmic intermediate domain γ and the catalytic domain α of DsbD (Figure 1) (Katzen and Beckwith, 2000; Goldstone et al., 2001). Therefore, CcdA, a much smaller and simpler polypeptide than DsbD, is sufficient for transmembrane electron transport.
Comparable thioreduction pathways for cytochrome c biogenesis have been suggested for R.capsulatus and E.coli. In these organisms, a membrane-tethered polypeptide with a Cys-Xa-Xb-Cys motif (Ccl2 and CcmH in R.capsulatus and E.coli, respectively) mediates electron transfer from a membrane-bound thioredoxin-like protein (HelX/CcmG) to c-type apocytochromes (Monika et al., 1997; Fabianek et al., 1999). Since: (i) DsbD interacts directly with CcmG (Katzen and Beckwith, 2000); and (ii) both CcdA and DsbD rely on either CcmG or its homolog HelX for cytochrome c biogenesis irrespective of the host, it is reasonable to propose that HelX is a periplasmic substrate of CcdA in R.capsulatus.
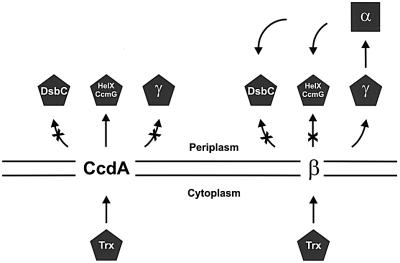
Our data suggest that CcdA and the central hydrophobic domain of DsbD, β, catalyze equivalent reactions, using thioredoxin-like proteins as substrates on each side of the membrane. Cytoplasmic thioredoxin appears to be the electron donor for both proteins. However, thioredoxin-like electron acceptors are different for CcdA and the β domain of DsbD, the former acting directly on HelX/CcmG and the latter directly on γ, the thioredoxin domain of DsbD itself (Figure 6). Therefore, CcdA and β could be considered as thioredoxin-oxidoreductases, with different substrate specificities. Interestingly, the periplasmic substrates are all tethered to the membrane in their wild-type state. This property might reflect an evolutionary strategy to increase their local concentration with respect to the transmembrane electron transporter core.
Fig. 6. Model showing the electron transport via CcdA and DsbD. The arrows show the electron flow. For simplicity, HelX and CcmG are represented by the same object, only CcdA and the central domain β are shown embedded in the membrane, and DsbG is not included in the scheme. Pentagons represent thioredoxin-like proteins, and the square represents an Ig-fold.
We propose to divide this electron transporter group of proteins into two families. The CcdA family, composed of polypeptides consisting of the hydrophobic central core, and the modular DsbD family, which includes those proteins that contain the central hydrophobic core plus the thioredoxin-like (γ) and Ig-like (α) periplasmic domains. While dsbD-like gene products are very homogeneous in size and in number of predicted TM segments, members of the CcdA family exhibit a wide variety of sizes with considerable heterogeneity at their N- and C-termini. It would be interesting to analyze whether among these varieties there are proteins with hitherto unknown functions.
The fact that CcdA, which corresponds to the central hydrophobic domain β, is sufficient for transferring reducing potential across the membrane raises intriguing questions about the need for the additional periplasmic domains of DsbD, α and γ. A phylogenetic analysis sheds some light on this problem. While dsbD is restricted to a few subdivisions of proteobacteria, ccdA-like genes are much more widespread (Figure 5B). Consequently, it is tempting to speculate that CcdA had evolved into DsbD by acquiring additional domains, rather than a reverse process in which DsbD had evolved into CcdA by losing such domains.
Overall, our data carry two fundamental implications. First, the periplasmic domains α and γ may function together as an adaptor module that allows the transmembrane electron transporter core to expand its substrate range (Figure 6). This provides DsbD with the advantage of branching the electron flow into two separate pathways: the cytochrome c thioreduction pathway, and the disulfide bond isomerization pathway. Secondly, since reduction of DsbC relies on the adaptor module of DsbD, which is absent on CcdA, and both DsbC and DsbD share a similar phylogenetic profile, these two proteins may have been the product of a co-evolutionary process that resulted in the emergence of the disulfide bond isomerization activity.
The former implication raises another intriguing question. Why does this adaptor module consist of two separate structures α and γ, instead of a single thioredoxin-like domain? While an expanding number of oxidative pathways involve direct interactions between thioredoxin-like and non-thioredoxin-like folds (Kadokura and Beckwith, 2001), there are no examples in the literature where electrons are transferred from the active site of one thioredoxin-like fold to another. Although the structural basis of this observation is unclear, the presence of α, a non-thioredoxin-like domain, might be required as an electron carrier between two thioredoxin-like proteins such as γ and the DsbD periplasmic electron acceptors DsbC, DsbG and CcmG.
Our results indicate that β is not capable of reducing CcmG/HelX. This finding suggests that, in the evolutionary process, while acquiring substrate specificity for γ, the hydrophobic core, formerly CcdA, lost the ability to interact with CcmG/HelX. The diversion of the electron flow through the periplasmic domains in DsbD probably relaxed the selective pressure that maintained the former functional link. Since a truncated CcdA variant lacking its last two C-terminal TM segments retains full activity (Deshmukh et al., 2000), it is conceivable that the substrate specificity of the central hydrophobic core might be imposed by the sequence of the first four TM segments. However, this hypothesis deserves further study.
Pairs of interacting proteins can have homologs in other organisms where they are fused into a single chain (Eisenberg et al., 2000). The evolutionary emergence of the modular structure has improved the efficiency of older enzymes and has given rise to a remarkable diversity of proteins. For example, different domain composition in eukaryotic protein disulfide isomerases results in a vast variety of proteins with diverse roles (Ferrari and Söling, 1999; Frand et al., 2000). However, reactions catalyzed by multimodular proteins are frequently more complex to study. Our findings on the functional relationship between DsbD and CcdA suggest that CcdA may present an alternative and simpler model for studying the molecular mechanism of this unusual transmembrane electron transfer.
Materials and methods
Strains and growth conditions
Strains used in this work are listed in Table I.
Table I. Strains and plasmids used in this work.
| Strain or plasmid | Relevant genotype or features | Source or reference |
|---|---|---|
| E.coli strains | ||
| MC1000 | araD139 (araABC-leu)7679 galU galK Δ(lac)X74 rpsL thi | Laboratory collection |
| S17-1 | thi RP4-2-Tc::Mu-Km::Tn7 | Simon et al. (1983) |
| AD494 | trxB::Kanr | Derman et al. (1993) |
| FED386 | MC1000 ΔdsbD napH::araC Pbad | Katzen and Beckwith (2000) |
| FED387 | FED386 ΔccmG zeh-298::Tn10 | Katzen and Beckwith (2000) |
| FED762 | FED386 trxB::Kanr | This work |
| R.capsulatus strains | ||
| MT1131 | Wild-type Nadi+, Ps+ | Scolnik et al. (1980) |
| ΔhelX | ΔhelX::Kanr | Beckman and Kranz (1993) |
| MD1 | MT1131 ΔccdA::Sper | Deshmukh et al. (2000) |
| MD10 | MT1131 ΔccdA::Sper, ΔhelX::Kanr | This work |
| Plasmids | ||
| pBAD18 | Arabinose regulation, pBR-based, ampicillinr | Guzman et al. (1995) |
| pBAD33 | Arabinose regulation, pACYC184-based, chloramphenicolr | Guzman et al. (1995) |
| pBAD43 | Arabinose regulation, pSC101-based, spectinomycinr | Stewart et al. (1999) |
| pRK415 | RK2-based, tetracycliner, oriT(mob+) | Keen et al. (1988) |
| pCHB500 | pRK415 with the cycA promoter region | Benning and Somerville (1992) |
| pHX1 | pRK415 with a DNA fragment containing the ΔhelX::Kanr allelle | This work |
| pFK017 | pBAD43 with APss-γ | Katzen and Beckwith (2000) |
| pFK049 | pBAD18 with αβ | Katzen and Beckwith (2000) |
| pFK051 | pBAD33 with α | Katzen and Beckwith (2000) |
| pFK060 | pBAD18 with β-c-Myc | Katzen and Beckwith (2000) |
| pFK093 | pBAD18 with DsbD | Katzen and Beckwith (2000) |
| pFK094 | pBAD18 with βγ | Katzen and Beckwith (2000) |
| pFK150 | pCHB500 with DsbD | This work |
| pFK154 | pCHB500 with αβ | This work |
| pFK156 | pCHB500 with βγ | This work |
| pFK169 | pBAD18 with CcdA-c-Myc | This work |
| pFK192 | pBAD33 with HelX-HA | This work |
| pFK211 | pRK415 with DsbD | This work |
| pFK213 | pCHB500 with β-c-Myc | This work |
| pFK225 | pCHB500 with CcdA-c-Myc | This work |
Escherichia coli cells were grown in NZ-amine (Rietsch et al., 1996) at 37°C, with the appropriate antibiotics. Rhodobacter capsulatus strains were grown chemoheterotrophically (aerobically) or photoheterotrophic ally (in anaerobic jars) in enriched medium (MPYE) or in Sistrom’s minimal medium as described previously (Deshmukh et al., 2000). Strain FED386 and its derivatives express the genes napB, napC and the ccm region upon induction with arabinose from the PBAD promoter (Katzen and Beckwith, 2000).
To construct the strain MD10, a fragment containing the ΔhelX::Kanr allele was PCR amplified from the chromosome of strain ΔhelX using primers DHelX_Xbafor (5′-GGAGGTCTAGAGCGGCCTTATTCATC TGCAG-3′) and DHelX_Xbarev (5′-GGAGGTCTAGATGTGCCGAC GCTGATCTTTG-3′), and cloned into pRK415. The resulting plasmid, pHX1, was verified by DNA sequencing. Gene replacement on strain MD1 was performed using the gene transfer agent as described previously (Yen et al., 1979).
Plasmid constructions
The plasmids used in this work are listed in Table I.
Plasmids pFK169 and pFK192 encoding CcdA-C-myc and HelX-hemagglutinin (HA) epitope were constructed by amplifying fragments from R.capsulatus MT1131 chromosome using the oligonucleotides pairs CcdA-F (5′-GAGGGTACCACACGGAGACACAGATTACCTCTCAT GACGCCGACTGACCTTCTCTTCGACGCG-3′) and CcdA_Myc-B (5′-CTCTCTAGACTACAGCAGATCTTCTTCAGAAATCAGTTTCT GTTCGCCCAGCTCCGCCAGCGCCGGGAAGGTGTCAAGCAGC-3′); and HelXF (5′-GAGGGTACCACACGGAGACACAGATTACCTCTCA TGGCTAAGCCCTTGATGTTTCTTCCGCTGCTGGTGATGGCGGGC TTTGTTGGC-3′) and HelX-HA-B (5′-CCTCTCTAGACTACGCCGC ATAGTCCGGAACATCGTACGGGTAAAGCTCGAGTGCGGCCGTCC CGGCCAGAAGCGGGTCGATCTTTTTCGTGATCACATCC-3′), re spectively. The products were digested with KpnI and XbaI and cloned into the corresponding sites of pBAD18 and pBAD33, respectively. In both cases, the genes are preceded by the untranslated upstream region of E.coli dsbD. All the constructions were verified by DNA sequencing. Genes encoding CcdA or DsbD variants were cloned into the broad host range vectors pRK415 or pCHB500 by excising the fragments from the corresponding E.coli plasmids (Table I). Plasmids were mobilized into R.capsulatus cells by conjugation using the strain S17-1 (Simon et al., 1983).
Cytochrome, western blot and thiol-redox state analyses
In-gel visualization of c-type cytochromes was performed using 3,3′,5,5′-tetramethylbenzidine (Aldrich, Milwaukee, WI) and H2O2 as described previously (Stewart et al., 1999). On-plate cytochrome c oxidase activity was detected as indicated (Deshmukh et al., 2000), after growing the cells on minimal medium plates for 2–3 days. Free thiols were acid trapped and alkylated with the high molecular mass reagent AMS (Molecular Probes, Eugene, OR), and proteins were revealed by western blotting as described (Stewart et al., 1999). Antibodies were described previously (Katzen and Beckwith, 2000).
Sequence analyses
To retrieve DsbC and DsbD homologs specifically from the database, sequences corresponding to the N-terminal dimerization domain of DsbC (McCarthy et al., 2000; Sun and Wang, 2000; Bader et al., 2001) and the Ig-like α domain of DsbD (Katzen and Beckwith, 2000; Goldstone et al., 2001; P.W.Haebel, D.Goldstone, F.Katzen, J.Beckwith and P.Metcalf, submitted for publication) were used as queries. These fragments correspond to peptide sequences Asp21–Lys85 and Gly20–Pro164 of the precursor forms of E.coli DsbC (DDBJ/EMBL/GenBank accession No. P21892) and DsbD (DDBJ/EMBL/GenBank accession No. P36655), respectively. To retrieve CcdA homologs from the database, the sequence of CcdA from R.capsulatus (DDBJ/EMBL/GenBank accession No. AAF26218) was employed. Four iterations were performed in each case, employing PSI- and PHI-BLAST (Altschul et al., 1997) and the non-redundant protein database at NCBI (http://www.ncbi.nlm.nih.gov/entrez/). The alignments were performed in Clustal X (Thompson et al., 1997) using the default parameters. Phylogenetic trees were constructed using the PAUP* (4.0) package (Sinauer Associates, Sunderland, MA). Heuristic searches were run under the distance optimality criterion using mean character difference. The starting trees were obtained via the neighbor-joining method, and the branch-swapping algorithm was tree bisection–reconnection (TBR). Transmembrane segment prediction analyses were performed using TMPred (http://www.ch.embnet.org/software/TMPRED_form.html) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/) (Krogh et al., 2001), respectively. Organelle targeting sequences were predicted using TargetP (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al., 2000). The R.capsulatus genome data were obtained from the site www.integratedgenomics.com.
Acknowledgments
Acknowledgements
Strain ΔhelX was kindly provided by Robert Kranz. We gratefully acknowledge members of the Beckwith laboratory, particularly Daniel Ritz, for stimulating discussions. We also thank Terri Luna for medium preparation, Ann McIntosh for administrative assistance, and Andrea Sequeira for help with phylogenetic analyses. F.K. is a Charles A. King Trust fellow. J.B. is an American Cancer Society Research Professor. This work was supported by NIH grant GM55090 to J.B. and DOE grant FG0291ER20052 to F.D.
References
- Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund F. and Beckwith,J. (1999) The thioredoxin superfamily: redundancy, specificity and gray-area genomics. J. Bacteriol., 181, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M.W., Hiniker,A., Regeimbal,J., Goldstone,D., Haebel,P.W., Riemer,J., Metcalf,P. and Bardwell,J.C. (2001) Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA. EMBO J., 20, 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J.C., McGovern,K. and Beckwith,J. (1991) Identification of a protein required for disulfide bond formation in vivo. Cell, 67, 581–589. [DOI] [PubMed] [Google Scholar]
- Bardwell J.C., Lee,J.O., Jander,G., Martin,N., Belin,D. and Beckwith,J. (1993) A pathway for disulfide bond formation in vivo. Proc. Natl Acad. Sci. USA, 90, 1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D.L. and Kranz,R.G. (1993) Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc. Natl Acad. Sci. USA, 90, 2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C. and Somerville,C.R. (1992) Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J. Bacteriol., 174, 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette P.H., Cotto,J.J., Gilbert,H.F. and Georgiou,G. (1999) In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J. Biol. Chem., 274, 7784–7792. [DOI] [PubMed] [Google Scholar]
- Chung J., Chen,T. and Missiakas,D. (2000) Transfer of electrons across the cytoplasmic membrane by DsbD, a membrane protein involved in thiol-disulphide exchange and protein folding in the bacterial periplasm. Mol. Microbiol., 35, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Crooke H. and Cole,J. (1995) The biogenesis of c-type cytochromes in Escherichia coli requires a membrane-bound protein, DipZ, with a protein disulphide isomerase-like domain. Mol. Microbiol., 15, 1139–1150. [DOI] [PubMed] [Google Scholar]
- Darby N.J., Raina,S. and Creighton,T.E. (1998) Contributions of substrate binding to the catalytic activity of DsbC. Biochemistry, 37, 783–791. [DOI] [PubMed] [Google Scholar]
- Derman A.I., Prinz,W.A., Belin,D. and Beckwith,J. (1993) Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science, 262, 1744–1747. [DOI] [PubMed] [Google Scholar]
- Deshmukh M., Brasseur,G. and Daldal,F. (2000) Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol. Microbiol., 35, 123–138. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Marcotte,E.M., Xenarios,I. and Yeates,T.O. (2000) Protein function in the post-genomic era. Nature, 405, 823–826. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O., Nielsen,H., Brunak,S. and von Heijne,G. (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol., 300, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Fabianek R.A., Hofer,T. and Thöny-Meyer,L. (1999) Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch. Microbiol., 171, 92–100. [DOI] [PubMed] [Google Scholar]
- Ferrari D.M. and Soling,H.D. (1999) The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J., 339, 1–10. [PMC free article] [PubMed] [Google Scholar]
- Frand A.R., Cuozzo,J.W. and Kaiser,C.A. (2000) Pathways for protein disulphide bond formation. Trends Cell Biol., 10, 203–210. [DOI] [PubMed] [Google Scholar]
- Goldstone D., Haebel,P.W., Katzen,F., Bader,M.W., Bardwell,J.C., Beckwith,J. and Metcalf,P. (2001) DsbC activation by the N-terminal domain of DsbD. Proc. Natl Acad. Sci. USA, 98, 9551–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.H., Page,M.D., Willis,A.C. and Ferguson,S.J. (2000) Escherichia coli DipZ: anatomy of a transmembrane protein disulphide reductase in which three pairs of cysteine residues, one in each of three domains, contribute differentially to function. Mol. Microbiol., 35, 1360–1374. [DOI] [PubMed] [Google Scholar]
- Guzman L.M., Belin,D., Carson,M.J. and Beckwith,J. (1995) Tight regulation, modulation and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol., 177, 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadokura H. and Beckwith,J. (2001) The expanding world of oxidative protein folding. Nat. Cell Biol., 3, E247–E249. [DOI] [PubMed] [Google Scholar]
- Katzen F. and Beckwith,J. (2000) Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell, 103, 769–779. [DOI] [PubMed] [Google Scholar]
- Keen N.T., Tamaki,S., Kobayashi,D. and Trollinger,D. (1988) Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene, 70, 191–197. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kishigami,S., Sone,M., Inokuchi,H., Mogi,T. and Ito,K. (1997) Respiratory chain is required to maintain oxidized states of the DsbA–DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc. Natl Acad. Sci. USA, 94, 11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A., Larsson,B., von Heijne,G. and Sonnhammer,E.L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol., 305, 567–580. [DOI] [PubMed] [Google Scholar]
- Martin J.L. (1995) Thioredoxin—a fold for all reasons. Structure, 3, 245–250. [DOI] [PubMed] [Google Scholar]
- McCarthy A.A., Haebel,P.W., Törronen,A., Rybin,V., Baker,E.N. and Metcalf,P. (2000) Crystal structure of the protein disulfide bond isomerase, DsbC, from Escherichia coli. Nat. Struct. Biol., 7, 196–199. [DOI] [PubMed] [Google Scholar]
- Missiakas D., Schwager,F. and Raina,S. (1995) Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J., 14, 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monika E.M., Goldman,B.S., Beckman,D.L. and Kranz,R.G. (1997) A thioreduction pathway tethered to the membrane for periplasmic cytochromes c biogenesis; in vitro and in vivo studies. J. Mol. Biol., 271, 679–692. [DOI] [PubMed] [Google Scholar]
- Page M.D., Saunders,N.F. and Ferguson,S.J. (1997) Disruption of the Pseudomonas aeruginosa dipZ gene, encoding a putative protein-disulfide reductase, leads to partial pleiotropic deficiency in c-type cytochrome biogenesis. Microbiology, 143, 3111–3122. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Assemat,K., Clément-Métral,J.D. and Klug,G. (1997) Thioredoxin is essential for Rhodobacter sphaeroides growth by aerobic and anaerobic respiration. Microbiology, 143, 83–91. [DOI] [PubMed] [Google Scholar]
- Potter L.C. and Cole,J.A. (1999) Essential roles for the products of the napABCD genes, but not napFGH, in periplasmic nitrate reduction by Escherichia coli K-12. Biochem. J., 344, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A., Belin,D., Martin,N. and Beckwith,J. (1996) An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc. Natl Acad. Sci. USA, 93, 13048–13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A., Bessette,P., Georgiou,G. and Beckwith,J. (1997) Reduction of the periplasmic disulfide bond isomerase, DsbC, occurs by passage of electrons from cytoplasmic thioredoxin. J. Bacteriol., 179, 6602–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz D. and Beckwith,J. (2001) Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol., 55, 21–48. [DOI] [PubMed] [Google Scholar]
- Schiött T. and Hederstedt,L. (2000) Efficient spore synthesis in Bacillus subtilis depends on the CcdA protein. J. Bacteriol., 182, 2845–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiött T., Throne-Holst,M. and Hederstedt,L. (1997a) Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J. Bacteriol., 179, 4523–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiött T., von Wachenfeldt,C. and Hederstedt,L. (1997b) Identification and characterization of the ccdA gene, required for cytochrome c synthesis in Bacillus subtilis. J. Bacteriol., 179, 1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P.A., Walker,M.A. and Marrs,B.L. (1980) Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem., 255, 2427–2432. [PubMed] [Google Scholar]
- Simon R., Priefer,U. and Pühler,A. (1983) A broad host range mobilization system for in vivo genetic-engineering—transposon mutagenesis in Gram-negative bacteria. Bio-Technology, 1, 784–791. [Google Scholar]
- Sone M., Akiyama,Y. and Ito,K. (1997) Differential in vivo roles played by DsbA and DsbC in the formation of protein disulfide bonds. J. Biol. Chem., 272, 10349–10352. [DOI] [PubMed] [Google Scholar]
- Stewart E.J., Katzen,F. and Beckwith,J. (1999) Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J., 18, 5963–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.X. and Wang,C.C. (2000) The N-terminal sequence (residues 1–65) is essential for dimerization, activities and peptide binding of Escherichia coli DsbC. J. Biol. Chem., 275, 22743–22749. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson,T.J., Plewniak,F., Jeanmougin,F. and Higgins,D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny-Meyer L. (2000) Haem–polypeptide interactions during cytochrome c maturation. Biochim. Biophys. Acta, 1459, 316–324. [DOI] [PubMed] [Google Scholar]
- Walker K.W. and Gilbert,H.F. (1997) Scanning and escape during protein-disulfide isomerase-assisted protein folding. J. Biol. Chem., 272, 8845–8848. [DOI] [PubMed] [Google Scholar]
- Yen H.C., Hu,N.T. and Marrs,B.L. (1979) Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J. Mol. Biol., 131, 157–168. [DOI] [PubMed] [Google Scholar]
- Zapun A., Missiakas,D., Raina,S. and Creighton,T.E. (1995) Structural and functional characterization of DsbC, a protein involved in disulfide bond formation in Escherichia coli. Biochemistry, 34, 5075–5089. [DOI] [PubMed] [Google Scholar]