Abstract
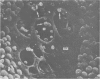
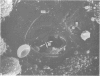
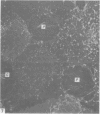
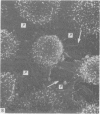
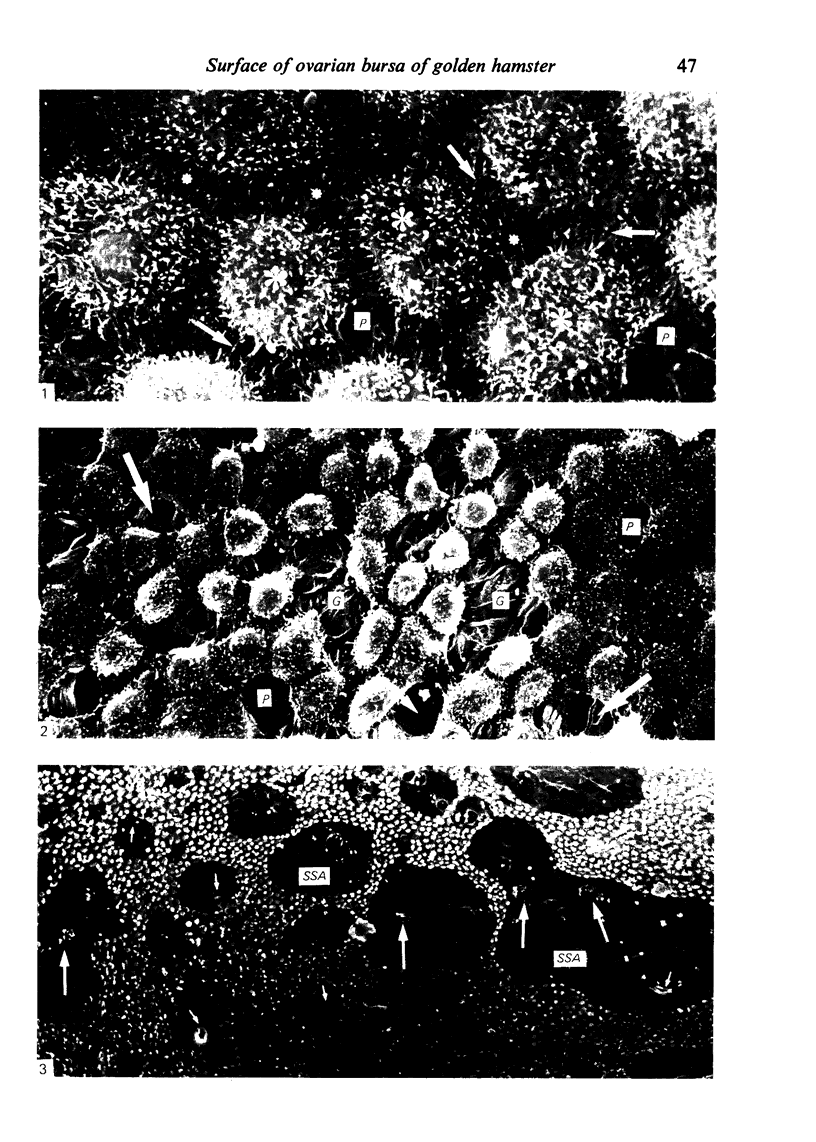
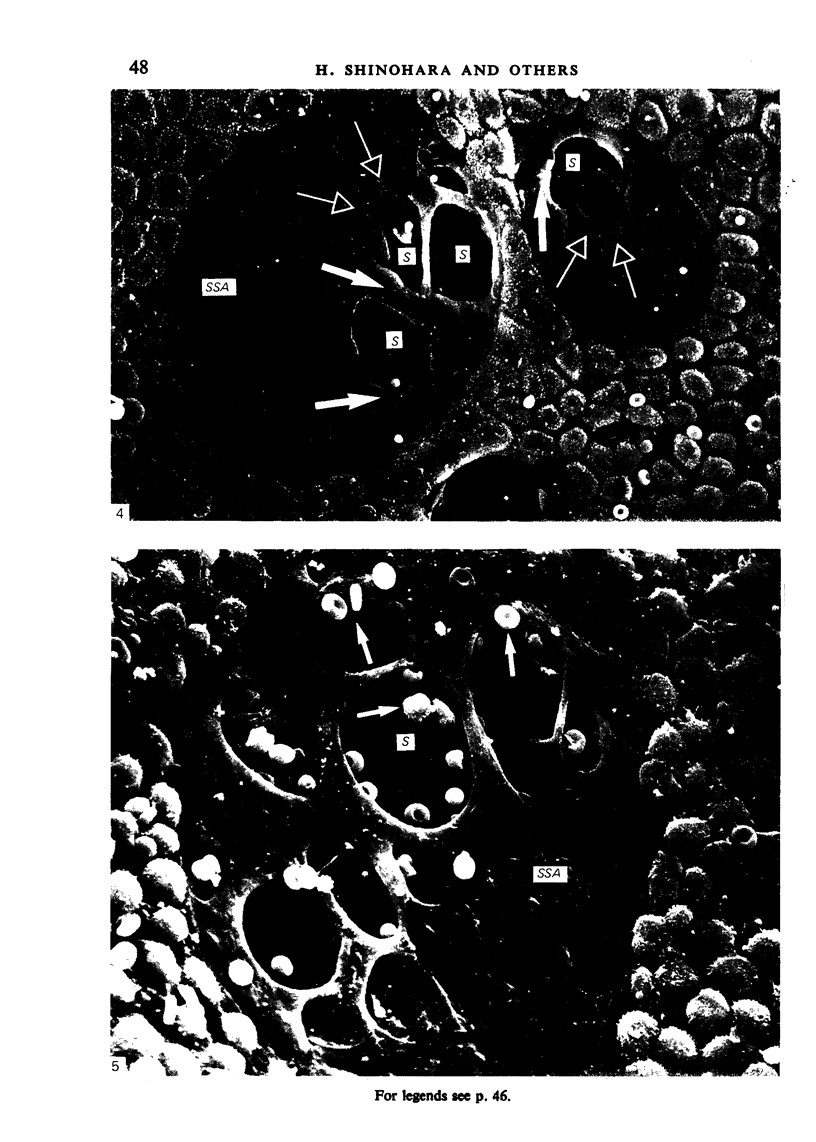
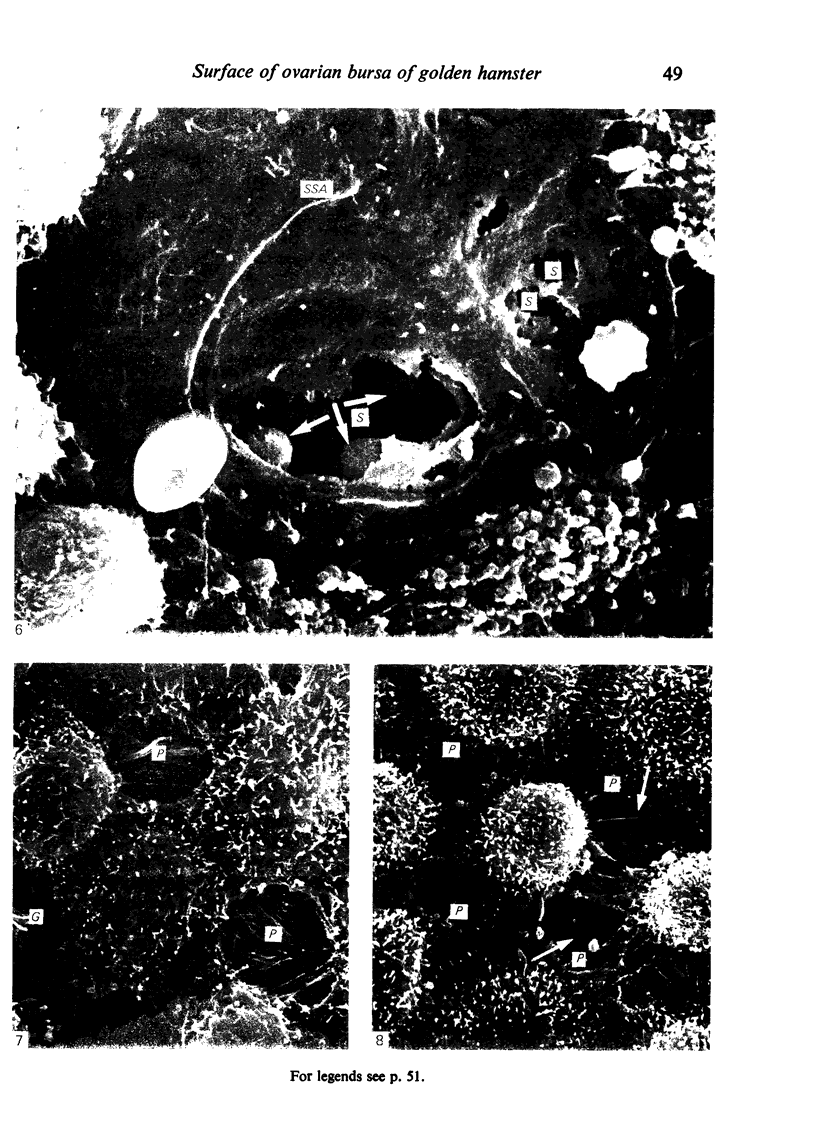
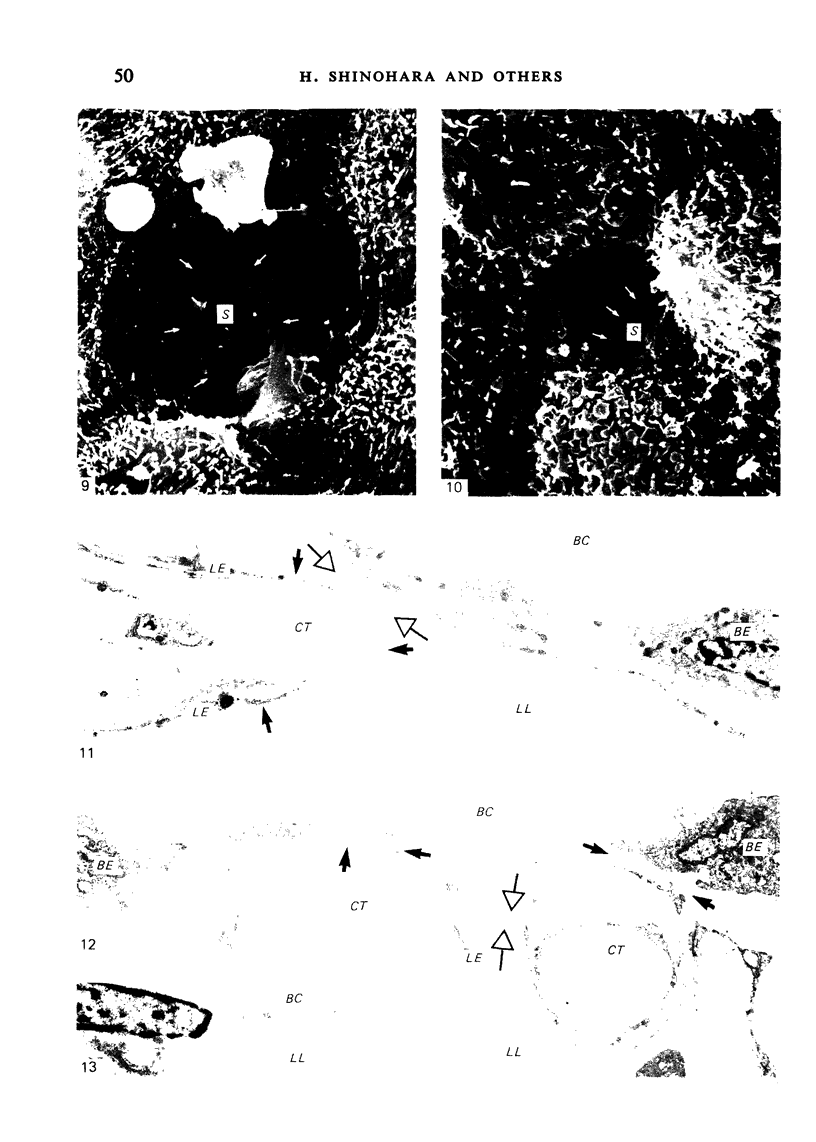
The inner surface of the ovarian bursa in the golden hamster was observed by scanning electron microscopy. There were numerous discontinuities in the bursal epithelium. Crevice-like gaps were formed between irregularly spaced epithelial cells, and the subepithelial connective tissue was exposed to the bursal cavity through the gaps. Through circular defects in the epithelial lining or pores, which were less than 20 microns in diameter, either the subepithelial connective tissue or stomatal orifices were visible. There were smooth surfaced areas lined with lymphatic endothelium, instead of bursal epithelium, which was continuous from the wall of the lymphatic vessel to the inner surface of the bursa. These areas were not present in all bursae, but if they were present, stomatal orifices consistently opened in them. Stomata in the ovarian bursa had two types of orifices, (1) circular orifices opening predominantly in smooth surfaced areas and measuring up to 50 microns in diameter; (2) jagged orifices opening usually in pores and measuring less than 10 microns in diameter. Blood cell components derived from ovulation entered lymphatics via stomata. Bursal fluid and small particles may drain into lymphatics directly via stomata and indirectly by diffusion through gaps, pores and connective tissue. Judging from the structural appearance of the stomatal orifices, the degree of opening of jagged orifices may change in response to changes in the cavity, while circular orifices may be stable openings.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARADI A. F., HOPE J. OBSERVATIONS ON ULTRASTRUCTURE OF RABBIT MESOTHELIUM. Exp Cell Res. 1964 Mar;34:33–44. doi: 10.1016/0014-4827(64)90180-6. [DOI] [PubMed] [Google Scholar]
- Bettendorf U. Lymph flow mechanism of the subperitoneal diaphragmatic lymphatics. Lymphology. 1978 Sep;11(3):111–116. [PubMed] [Google Scholar]
- HARVEY E. B., YANAGIMACHI R., CHANG M. C. Onset of estrus and ovulation in the golden hamster. J Exp Zool. 1961 Apr;146:231–235. doi: 10.1002/jez.1401460303. [DOI] [PubMed] [Google Scholar]
- Leak L. V., Rahil K. Permeability of the diaphragmatic mesothelium: the ultrastructural basis for "stomata". Am J Anat. 1978 Apr;151(4):557–593. doi: 10.1002/aja.1001510409. [DOI] [PubMed] [Google Scholar]
- Martin G. G., Talbot P., Pendergrass P. An intrabursal injection procedure for the in vivo study of ovulation in hamsters. J Exp Zool. 1981 Jun;216(3):461–468. doi: 10.1002/jez.1402160315. [DOI] [PubMed] [Google Scholar]
- Nakatani T., Shinohara H., Takeda K., Morisawa S., Matsuda T. Morphology of the intercapsular segment of the oviduct in the golden hamster with special reference to ovum-transit from ruptured follicles to the ampulla. Experientia. 1985 Mar 15;41(3):368–370. doi: 10.1007/BF02004509. [DOI] [PubMed] [Google Scholar]
- Shinohara H., Nakatani T., Matsuda T. The presence of lymphatic stomata in the ovarian bursa of the golden hamster. Anat Rec. 1985 Sep;213(1):44–52. doi: 10.1002/ar.1092130107. [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C., Wissig S. L. Lymphatic absorption from the peritoneal cavity: regulation of patency of mesothelial stomata. Microvasc Res. 1983 Jan;25(1):22–39. doi: 10.1016/0026-2862(83)90041-9. [DOI] [PubMed] [Google Scholar]