Abstract
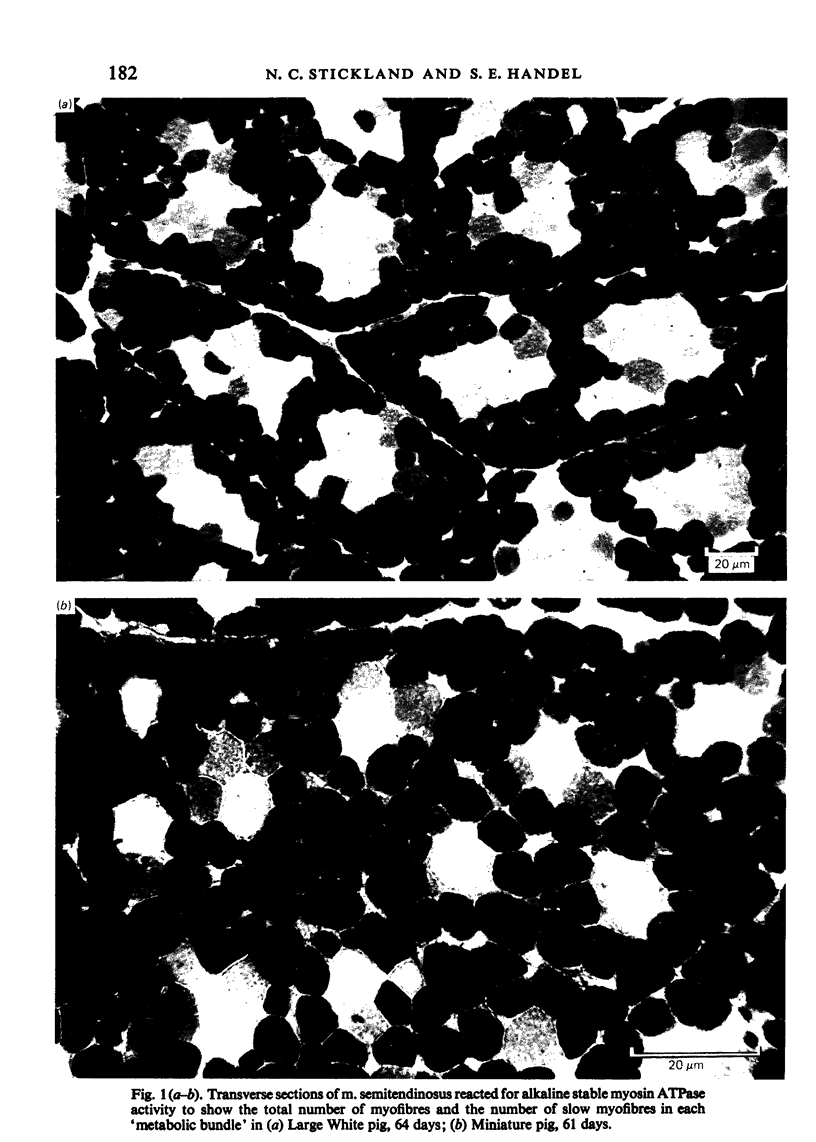
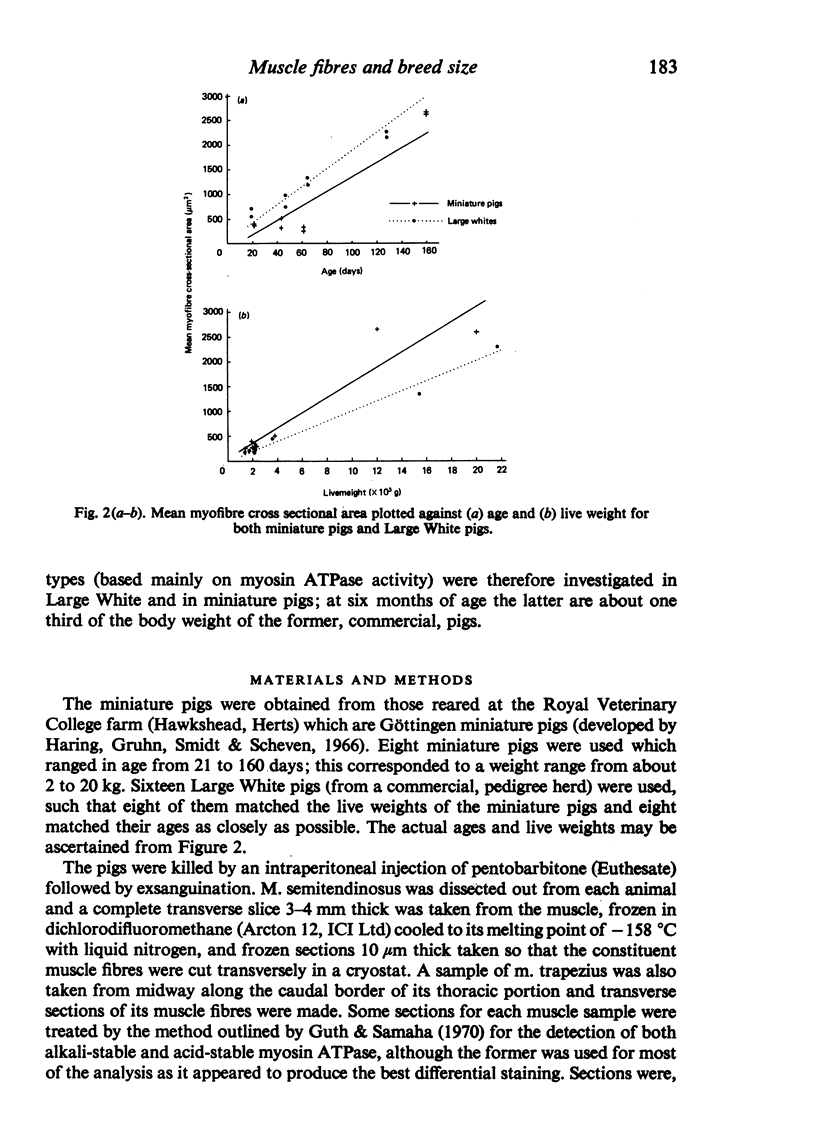
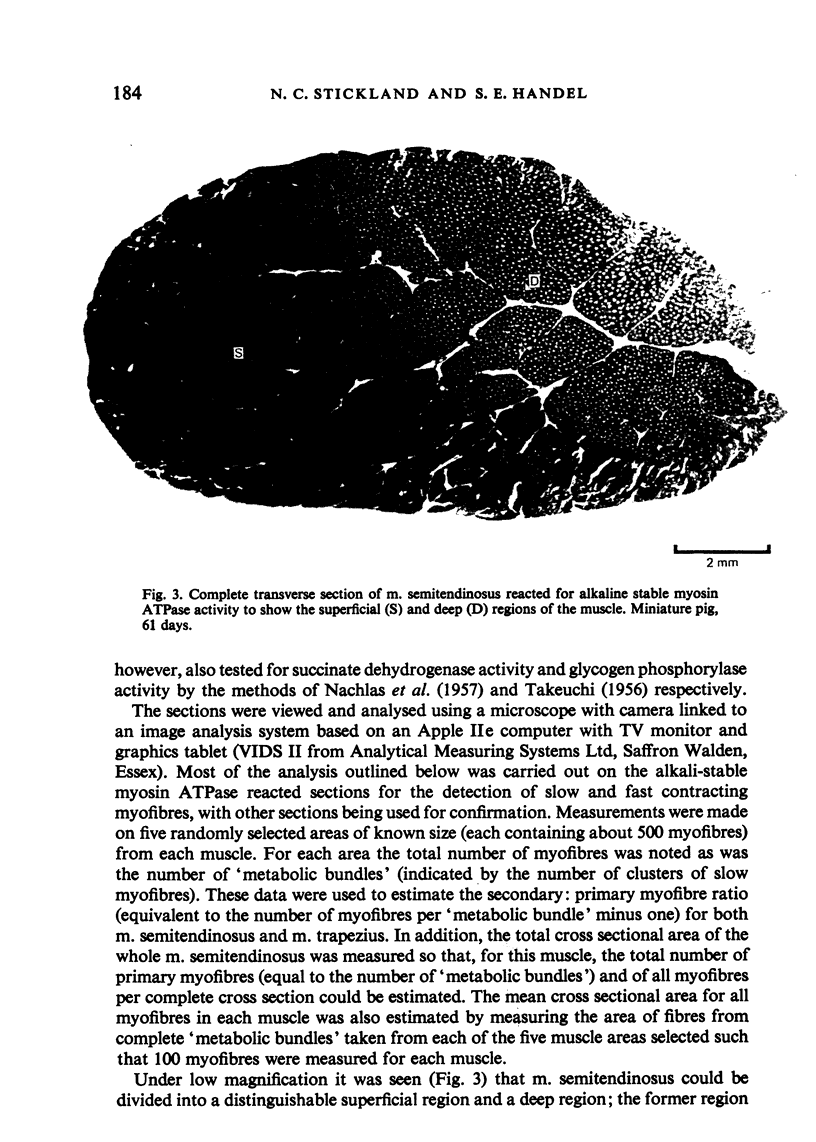
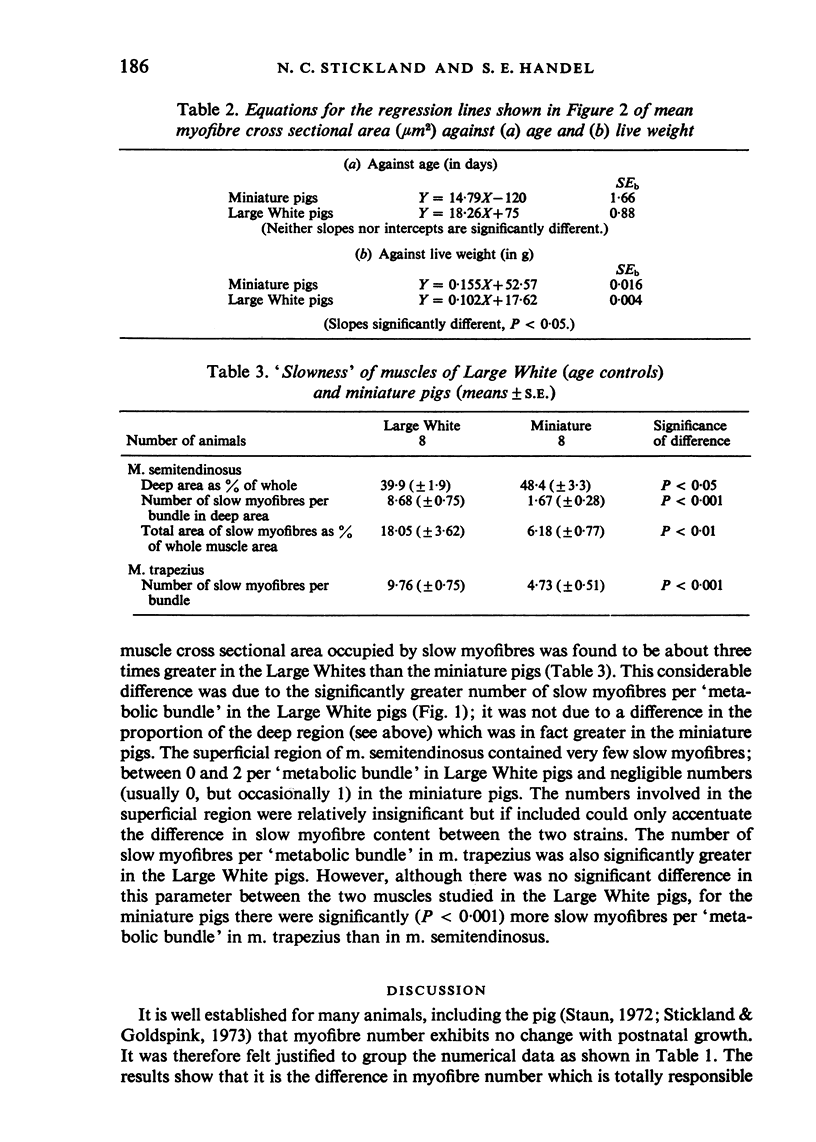
M. semitendinosus and m. trapezius (portion) were removed from eight miniature pigs ranging from 21 to 160 days of age and eight age-control as well as eight weight-control commercial Large White pigs. Complete transverse frozen sections were obtained for each muscle sample and stained for various enzyme activities including myosin adenosine triphosphatase activity which enabled the identification of 'metabolic bundles'. This in turn enabled conclusions to be made about the prenatal development of the muscle in terms of primary and secondary myofibres. The Large White pigs contained 173% more muscle fibres in m. semitendinosus than did the miniature pigs. Primary myofibre number was found to be about four times more important than secondary to primary myofibre ratios in determining myofibre number in the two breeds of pigs. Both primary myofibre number and secondary to primary myofibre ratios were, however, significantly greater in Large White than in miniature pigs. When the age- and weight-control Large Whites were compared with the miniature pigs it was found that at any given live weight the miniature pigs had thicker myofibres whereas at the same age there was no significant difference. The total area of m. semitendinosus occupied by slow myofibres was about three times greater in the Large White pigs; the functional aspects of this are discussed. It was concluded that genetically smaller animals develop fewer muscle fibres in their muscles by a different mechanism to that exhibited by animals which are smaller due to nutritional deprivation in utero.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore C. R., Addis P. B., Doerr L. Development of muscle fibers in the fetal pig. J Anim Sci. 1973 Jun;36(6):1088–1093. doi: 10.2527/jas1973.3661088x. [DOI] [PubMed] [Google Scholar]
- Bedi K. S., Birzgalis A. R., Mahon M., Smart J. L., Wareham A. C. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br J Nutr. 1982 May;47(3):417–431. doi: 10.1079/bjn19820053. [DOI] [PubMed] [Google Scholar]
- Beermann D. H., Cassens R. G., Hausman G. J. A second look at fiber type differentiation in porcine skeletal muscle. J Anim Sci. 1978 Jan;46(1):125–132. doi: 10.2527/jas1978.461125x. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Meadows D. A., Zernicke R. F., Sacks R. D., Fournier M., Edgerton V. R. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982 Jul;48(1):192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Davies A. S. Postnatal changes in the histochemical fibre types of procine skeletal muscle. J Anat. 1972 Nov;113(Pt 2):213–240. [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Hegarty P. V., Allen C. E. Effect of pre-natal runting on the post-natal development of skeletal muscles in swine and rats. J Anim Sci. 1978 Jun;46(6):1634–1640. doi: 10.2527/jas1978.4661634x. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Goldspink G. Large and small muscles. Life Sci. 1967 Sep 1;6(17):1821–1826. doi: 10.1016/0024-3205(67)90210-x. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Powell S. E., Aberle E. D. Skeletal muscle and adipose tissue cellularity in runt and normal birth weight swine. J Anim Sci. 1981 Apr;52(4):748–756. doi: 10.2527/jas1981.524748x. [DOI] [PubMed] [Google Scholar]
- Pullen A. H. The distribution and relative sizes of three histochemical fibre types in the rat tibialis anterior muscle. J Anat. 1977 Feb;123(Pt 1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Robinson D. W. The cellular response of porcine skeletal muscle to prenatal and neonatal nutritional stress. Growth. 1969 Sep;33(3):231–240. [PubMed] [Google Scholar]
- Swatland H. J., Cassens R. G. Prenatal development, histochemistry and innervation of porcine muscle. J Anim Sci. 1973 Feb;36(2):343–354. doi: 10.2527/jas1973.362343x. [DOI] [PubMed] [Google Scholar]
- Wigmore P. M., Stickland N. C. Muscle development in large and small pig fetuses. J Anat. 1983 Sep;137(Pt 2):235–245. [PMC free article] [PubMed] [Google Scholar]