Abstract
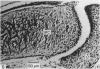
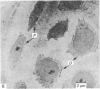
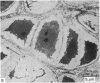
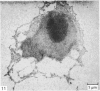
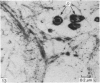
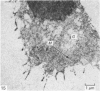
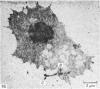
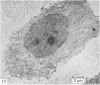
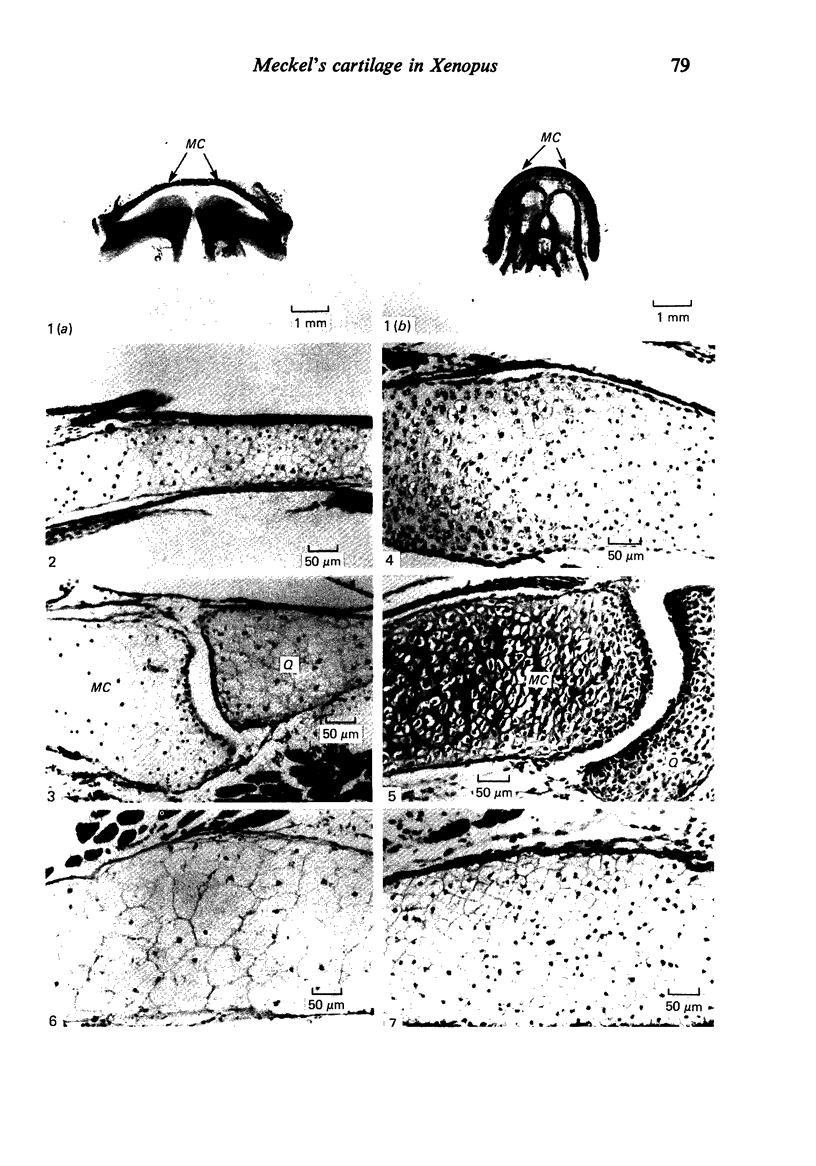
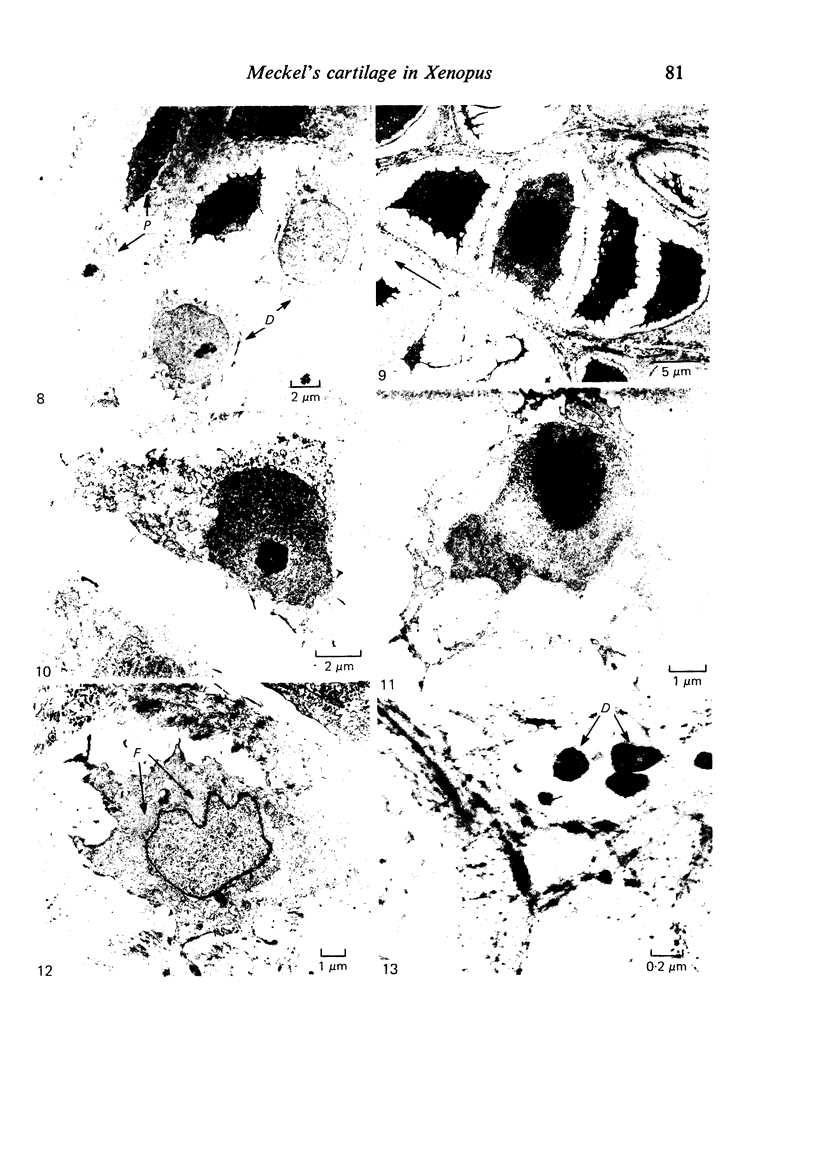
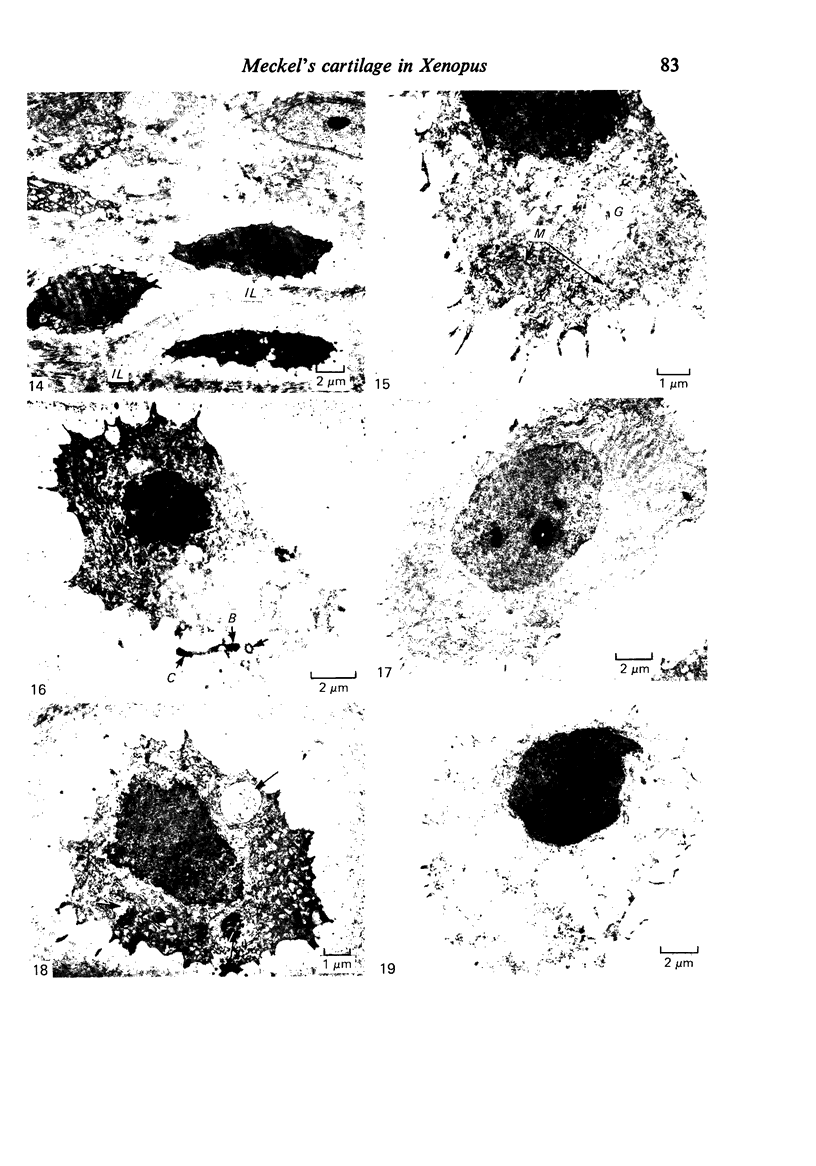
Meckel's cartilage, in Xenopus laevis prior to metamorphosis, is a tissue exhibiting very large lacunae, separated by thin rims of matrix, presenting a net-like appearance, similar to that of cartilage in invertebrates. The cells on the periphery of the tissue are rather more flattened, and more closely packed. On the lateral aspects of the cartilage distinct columns of apparently dividing cells are evident. During metamorphic climax, the amount of matrix separating the lacunae increases, with an associated decrease in lacunar size, and some of the deeper cells develop cilia, which are not seen either before or after climax. By the end of metamorphic climax there is a considerable increase in the amount of matrix present in the tissue, while many cells at all depths in the cartilage show the presence of lysosome-like structures, possibly associated with the changing shape of the cartilage. Intramembranous ossification is proceeding around Meckel's cartilage, but there is no evidence of endochondral ossification up to the end of metamorphosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNETT C. H., COCHRANE W., PALFREY A. J. AGE CHANGES IN ARTICULAR CARTILAGE OF RABBITS. Ann Rheum Dis. 1963 Nov;22:389–400. doi: 10.1136/ard.22.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesterman P. J., Smith A. U. Homotransplantation of articular cartilage and isolated chondrocytes. An experimental study in rabbits. J Bone Joint Surg Br. 1968 Feb;50(1):184–197. [PubMed] [Google Scholar]
- DAEMS W. T., van RIJSSEL T. The fine structure of the peribiliary dense bodies in mouse liver tissue. J Ultrastruct Res. 1961 Jun;5:263–290. doi: 10.1016/s0022-5320(61)90020-x. [DOI] [PubMed] [Google Scholar]
- Dickson G. R. Ultrastructure of growth cartilage in the proximal femur of the frog, Rana temporaria. J Anat. 1982 Oct;135(Pt 3):549–564. [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T. The secretion of enzymes into the pericellular environment. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):315–324. doi: 10.1098/rstb.1975.0055. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Thomas I., Yong N., Lalonde J. M. Ultrastructure of rabbit semilunar cartilages. J Anat. 1978 Mar;125(Pt 3):499–517. [PMC free article] [PubMed] [Google Scholar]
- Meachim G., Roy S. Intracytoplasmic filaments in the cells of adult human articular cartilage. Ann Rheum Dis. 1967 Jan;26(1):50–58. doi: 10.1136/ard.26.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person P., Philpott D. E. On the occurrence and the biologic significance of cartilage tissues in invertebrates. Clin Orthop Relat Res. 1967 Jul-Aug;53:185–212. [PubMed] [Google Scholar]
- Scherft J. P., Daems W. T. Single cilia in chondrocytes. J Ultrastruct Res. 1967 Aug 30;19(5):546–555. doi: 10.1016/s0022-5320(67)80080-7. [DOI] [PubMed] [Google Scholar]
- Sprinz R., Stockwell R. A. Changes in articular cartilage following intraarticular injection of tritiated glyceryl trioleate. J Anat. 1976 Sep;122(Pt 1):91–112. [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971 Sep;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]
- Wilsman N. J. Cilia of adult canine articular chondrocytes. J Ultrastruct Res. 1978 Sep;64(3):270–281. doi: 10.1016/s0022-5320(78)90036-9. [DOI] [PubMed] [Google Scholar]
- Wislman N. J., Fletcher T. F. Cilia of neonatal articular chondrocytes: incidence and morphology. Anat Rec. 1978 Apr;190(4):871–889. doi: 10.1002/ar.1091900408. [DOI] [PubMed] [Google Scholar]