Abstract
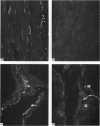
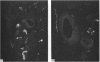
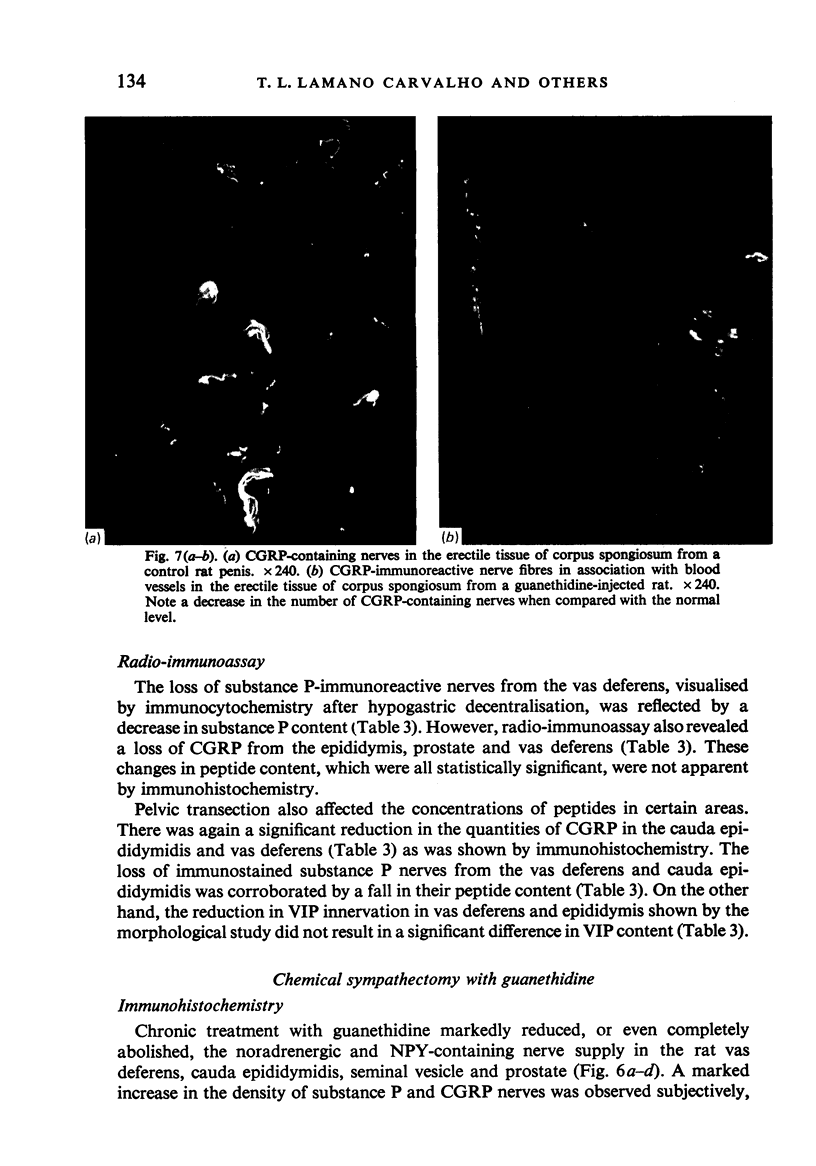
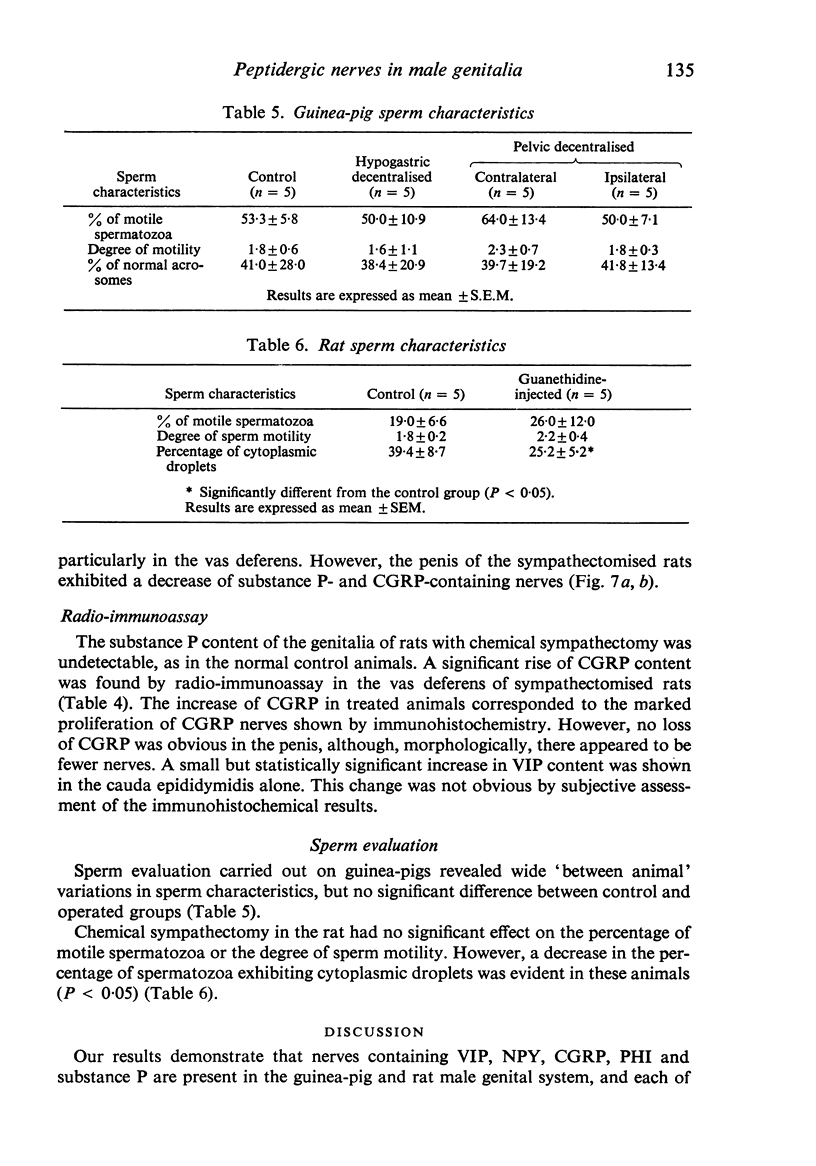
A systematic immunohistochemical and radio-immunological survey of the occurrence, distribution and origin of the peptidergic nerve supply in guinea-pig and rat male genitalia is presented. Neuropeptide Y (NPY), vasoactive intestinal polypeptide (VIP), peptide histidine isoleucine (PHI), substance P and CGRP were detected in the genital organs of both species. The densities and distribution patterns of the peptidergic nerves were compared with those of the adrenergic nerves, as revealed by antibodies raised against dopamine-beta-hydroxylase (D beta H) and tyrosine hydroxylase (TH), and the general neuronal component, as revealed by antibodies raised against neurofilament proteins (NF). Bilateral transection of the hypogastric nerves, in the guinea-pig, resulted in a decrease of substance P-containing nerves in the vas deferens and of NPY-, PHI- and VIP-containing nerves in the seminal vesicle. Unilateral disconnection of the pelvic nerves caused a decrease of VIP, PHI, substance P and CGRP nerve supply in the ipsilateral vas deferens and cauda epididymidis in the guinea-pig. A marked reduction of noradrenergic and NPY-containing nerves was observed in the vas deferens and sexual accessory glands of rats, chemically sympathectomised by chronic injection of low doses of guanethidine. Conversely, increase of substance P and CGRP immunoreactivities were observed, particularly in the vas deferens. After guanethidine, the cauda epididymidis and vas deferens were distended with spermatozoa, suggesting paralysis of the ducts. Spermatozoa had a decreased percentage of attached cytoplasmic droplets, indicating prolonged retention in the ducts.
Full text
PDF

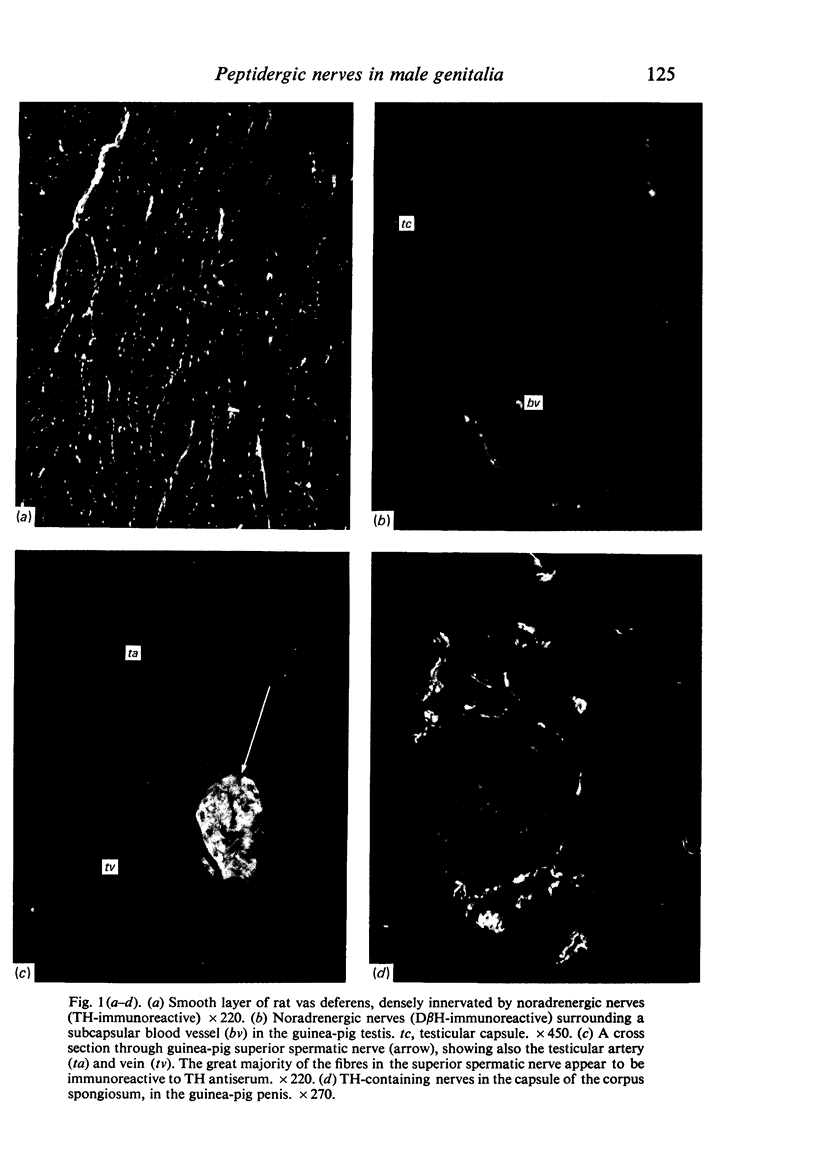

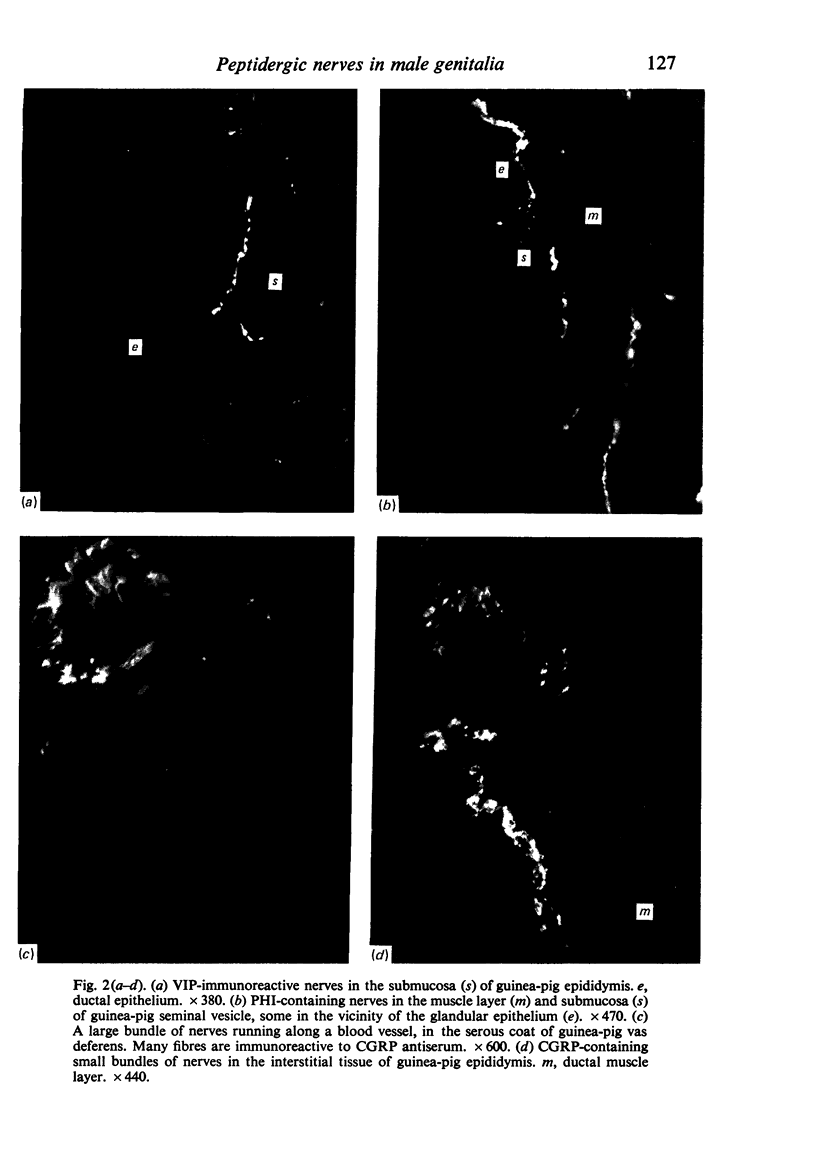
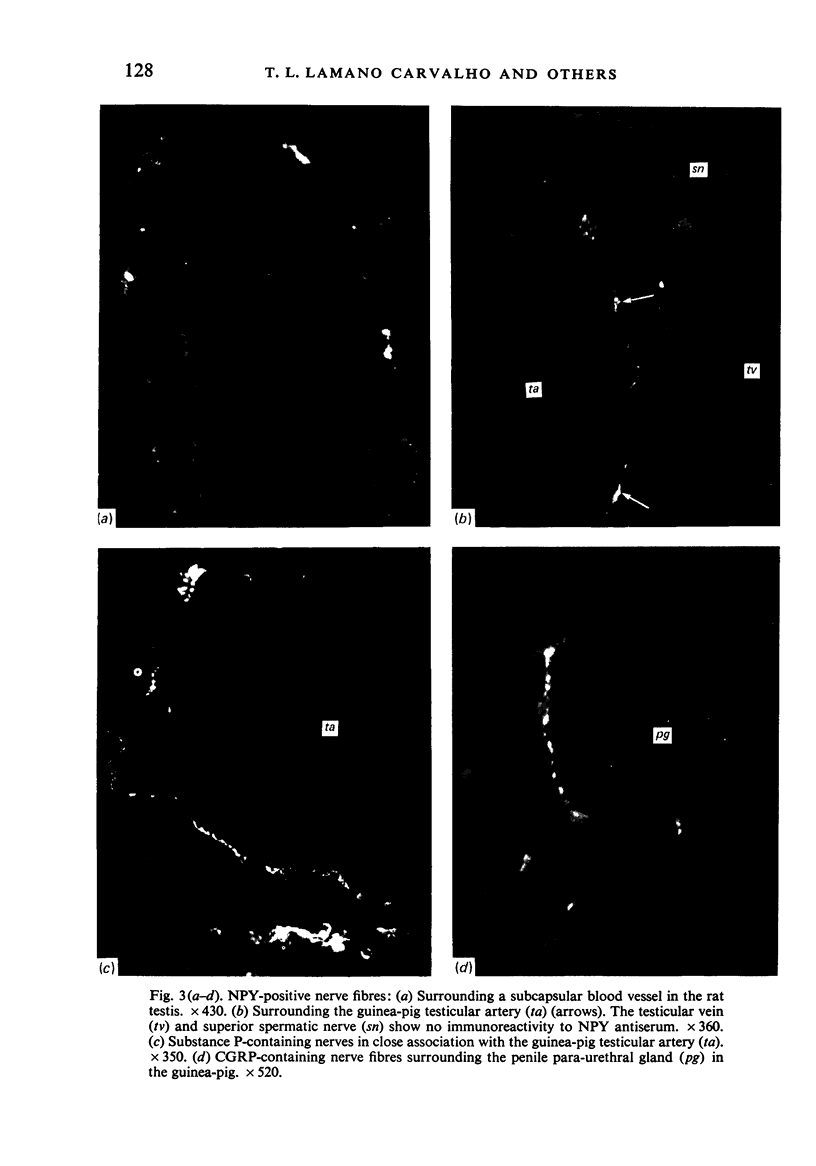



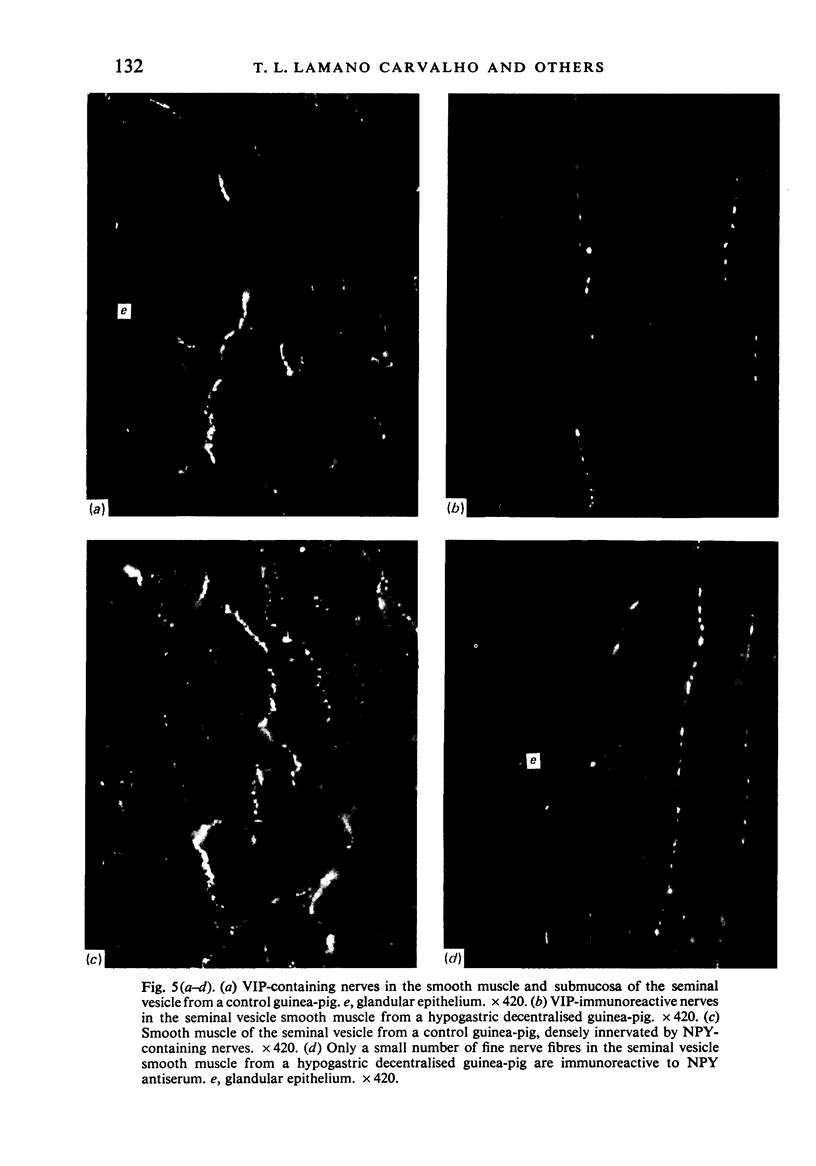







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Gu J., Allen J. M., Tatemoto K., Polak J. M., Bloom S. R. Neuropeptide Y in the human male genital tract. Life Sci. 1984 Dec 24;35(26):2643–2648. doi: 10.1016/0024-3205(84)90033-x. [DOI] [PubMed] [Google Scholar]
- Allen J. M., Adrian T. E., Tatemoto K., Polak J. M., Hughes J., Bloom S. R. Two novel related peptides, neuropeptide Y (NPY) and peptide YY (PYY) inhibit the contraction of the electrically stimulated mouse vas deferens. Neuropeptides. 1982 Dec;3(2):71–77. doi: 10.1016/0143-4179(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Alm P., Alumets J., Brodin E., Håkanson R., Nilsson G., Sjöberg N. O., Sundler F. Peptidergic (substance P) nerves in the genito-urinary tract. Neuroscience. 1978;3(4-5):419–425. doi: 10.1016/0306-4522(78)90044-1. [DOI] [PubMed] [Google Scholar]
- Alm P., Alumets J., Håkanson R., Sundler F. Peptidergic (vasoactive intestinal peptide) nerves in the genito-urinary tract. Neuroscience. 1977;2(5):751–754. doi: 10.1016/0306-4522(77)90028-8. [DOI] [PubMed] [Google Scholar]
- Bell C. Autonomic nervous control of reproduction: circulatory and other factors. Pharmacol Rev. 1972 Dec;24(4):657–736. [PubMed] [Google Scholar]
- Bishop A. E., Carlei F., Lee V., Trojanowski J., Marangos P. J., Dahl D., Polak J. M. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry. 1985;82(1):93–97. doi: 10.1007/BF00502095. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Yiangou Y., Christofides N. D., Bloom S. R. The distributions of PHI and VIP in porcine gut and their co-localisation to a proportion of intrinsic ganglion cells. Peptides. 1984 Mar-Apr;5(2):255–259. doi: 10.1016/0196-9781(84)90215-8. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Christofides N. D., Delamarter J., Buell G., Kawashima E., Polak J. M. Diarrhoea in vipoma patients associated with cosecretion of a second active peptide (peptide histidine isoleucine) explained by single coding gene. Lancet. 1983 Nov 19;2(8360):1163–1165. doi: 10.1016/s0140-6736(83)91215-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Evans B., Gannon B. J., Heath J. W., James V. A new method of destroying adrenergic nerves in adult animals using guanethidine. Br J Pharmacol. 1971 Oct;43(2):295–301. [PMC free article] [PubMed] [Google Scholar]
- COUJARD R. Contribution a l'étude des voies nerveuses sympathiques du testicule. Arch Anat Microsc Morphol Exp. 1954;43(4):321–364. [PubMed] [Google Scholar]
- CROSS B. A., GLOVER T. D. The hypothalamus and seminal emission. J Endocrinol. 1958 Apr;16(4):385–395. doi: 10.1677/joe.0.0160385. [DOI] [PubMed] [Google Scholar]
- Cole D. F., Bloom S. R., Burnstock G., Butler J. M., McGregor G. P., Saffrey M. J., Unger W. G., Zhang S. Q. Increase in SP-like immunoreactivity in nerve fibres of rabbit iris and ciliary body one to four months following sympathetic denervation. Exp Eye Res. 1983 Aug;37(2):191–197. doi: 10.1016/0014-4835(83)90078-7. [DOI] [PubMed] [Google Scholar]
- Dixson A. F., Kendrick K. M., Blank M. A., Bloom S. R. Effects of tactile and electrical stimuli upon release of vasoactive intestinal polypeptide in the mammalian penis. J Endocrinol. 1984 Feb;100(2):249–252. doi: 10.1677/joe.0.1000249. [DOI] [PubMed] [Google Scholar]
- Dott H. M., Dingle J. T. Distribution of lysosomal enzymes in the spermatozoa and cytoplasmic droplets of bull and ram. Exp Cell Res. 1968 Oct;52(2):523–540. doi: 10.1016/0014-4827(68)90493-x. [DOI] [PubMed] [Google Scholar]
- Emmens C. W. The motility and viability of rabbit spermatozoa at different hydrogen-ion concentrations. J Physiol. 1947 Oct 15;106(4):471–481. doi: 10.1113/jphysiol.1947.sp004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B., Gannon B. J., Heath J. W., Burnstock G. Long-lasting damage to the internal male genital organs and their adrenergic innervation in rats following chronic treatment with the antihypertensive drug guanethidine. Fertil Steril. 1972 Sep;23(9):657–667. doi: 10.1016/s0015-0282(16)39194-4. [DOI] [PubMed] [Google Scholar]
- Ghiglione M., Christofides N. D., Yiangou Y., Uttenthal L. O., Bloom S. R. PHI stimulates intestinal fluid secretion. Neuropeptides. 1982 Dec;3(2):79–82. doi: 10.1016/0143-4179(82)90002-6. [DOI] [PubMed] [Google Scholar]
- Gibson S. J., Polak J. M., Bloom S. R., Sabate I. M., Mulderry P. M., Ghatei M. A., McGregor G. P., Morrison J. F., Kelly J. S., Evans R. M. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci. 1984 Dec;4(12):3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Islam K. N., Polak J. M. Repeated application of first-layer antiserum improves immunofluorescence staining: a modification of the indirect immunofluorescence staining procedure. Histochem J. 1983 May;15(5):475–482. doi: 10.1007/BF01002701. [DOI] [PubMed] [Google Scholar]
- Gu J., Polak J. M., Probert L., Islam K. N., Marangos P. J., Mina S., Adrian T. E., McGregor G. P., O'Shaughnessy D. J., Bloom S. R. Peptidergic innervation of the human male genital tract. J Urol. 1983 Aug;130(2):386–391. doi: 10.1016/s0022-5347(17)51174-x. [DOI] [PubMed] [Google Scholar]
- HODSON N. ROLE OF THE HYPOGASTRIC NERVES IN SEMINAL EMISSION IN THE RABBIT. J Reprod Fertil. 1964 Feb;7:113–122. doi: 10.1530/jrf.0.0070113. [DOI] [PubMed] [Google Scholar]
- Heath J. W., Burnstock G. Selectivity of neuronal degeneration produced by chronic guanethidine treatment. J Neurocytol. 1977 Aug;6(4):397–405. doi: 10.1007/BF01178225. [DOI] [PubMed] [Google Scholar]
- Hodson N. Sympathetic nerves and reproductive organs in the male rabbit. J Reprod Fertil. 1965 Oct;10(2):209–220. doi: 10.1530/jrf.0.0100209. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Itoh N., Obata K., Yanaihara N., Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983 Aug 11;304(5926):547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bohn M. C., Black I. B. Substance P in principal sympathetic neurons: regulation by impulse activity. Science. 1981 Oct 16;214(4518):335–336. doi: 10.1126/science.6169153. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Bell W. O., Black I. B. Interactions between the sympathetic and sensory innervation of the iris. J Neurosci. 1983 Jun;3(6):1301–1307. doi: 10.1523/JNEUROSCI.03-06-01301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Comparative study of some isolated mammalian smooth muscle effectors of penile erection. Acta Physiol Scand. 1977 Jul;100(3):354–367. doi: 10.1111/j.1748-1716.1977.tb05961.x. [DOI] [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Suppression of the excitatory adrenergic neurotransmission; a possible role of cholinergic nerves in the retractor penis muscle. Acta Physiol Scand. 1977 Jul;100(3):368–376. doi: 10.1111/j.1748-1716.1977.tb05962.x. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part VII. Anatomical Observations. J Physiol. 1896 Oct 19;20(4-5):372–406. doi: 10.1113/jphysiol.1896.sp000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I. Occurrence of nerves containing vasoactive intestinal polypeptide immunoreactivity in the male genital tract. Life Sci. 1977 Aug 15;21(4):503–508. doi: 10.1016/0024-3205(77)90088-1. [DOI] [PubMed] [Google Scholar]
- Lindner G., Grosse G. Uber die Wirkung von Substanz P auf das Wachstum von Nervenfasern unter In-vitro-Bedingungen. Z Mikrosk Anat Forsch. 1981;95(3):390–394. [PubMed] [Google Scholar]
- Lundberg J. M., Terenius L., Hökfelt T., Martling C. R., Tatemoto K., Mutt V., Polak J., Bloom S., Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982 Dec;116(4):477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- McGregor G. P., Bloom S. R. Radioimmunoassay of substance P and its stability in tissue. Life Sci. 1983 Feb 7;32(6):655–662. doi: 10.1016/0024-3205(83)90211-4. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Gu J., Mina S., Bloom S. R. Vipergic nerves in the penis. Lancet. 1981 Aug 1;2(8240):217–219. doi: 10.1016/s0140-6736(81)90471-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Terenghi G., Polak J. M., Ghatei M. A., Mulderry P. K., Butler J. M., Unger W. G., Bloom S. R. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol. 1985 Mar 22;233(4):506–516. doi: 10.1002/cne.902330410. [DOI] [PubMed] [Google Scholar]
- Vaalasti A., Linnoila I., Hervonen A. Immunohistochemical demonstration of VIP, [Met5]-and [Leu5]-enkephalin immunoreactive nerve fibres in the human prostate and seminal vesicles. Histochemistry. 1980;66(1):89–98. doi: 10.1007/BF00493249. [DOI] [PubMed] [Google Scholar]
- Virag R., Ottesen B., Levy C., Wagner G. Vasoactive intestinal polypeptide release during penile erection in man. Lancet. 1982 Nov 20;2(8308):1166–1166. doi: 10.1016/s0140-6736(82)92828-8. [DOI] [PubMed] [Google Scholar]
- Watson P. F. Use of a Giemsa stain to detect changes in acrosomes of frozen ram spermatozoa. Vet Rec. 1975 Jul 5;97(1):12–15. doi: 10.1136/vr.97.1.12. [DOI] [PubMed] [Google Scholar]
- Zhang S. Q., Terenghi G., Unger W. G., Ennis K. W., Polak J. Changes in substance P- and neuropeptide Y-immunoreactive fibres in rat and guinea-pig irides following unilateral sympathectomy. Exp Eye Res. 1984 Sep;39(3):365–372. doi: 10.1016/0014-4835(84)90024-1. [DOI] [PubMed] [Google Scholar]