Abstract
In the Caenorhabditis elegans hermaphrodite germline, spatially restricted mitogen-activated protein kinase (MAPK) signalling controls the meiotic cell cycle. First, the MAPK signal is necessary for the germ cells to progress through pachytene of meiotic prophase I. As the germ cells exit pachytene and enter diplotene/diakinesis, MAPK is inactivated and the developing oocytes arrest in diakinesis (G2/M arrest). During oocyte maturation, a signal from the sperm reactivates MAPK to promote M phase entry. Here, we show that the MAPK phosphatase LIP-1 dephosphorylates MAPK as germ cells exit pachytene in order to maintain MAPK in an inactive state during oocyte development. Germ cells lacking LIP-1 fail to arrest the cell cycle at the G2/M boundary, and they enter a mitotic cell cycle without fertilization. LIP-1 thus coordinates oocyte cell cycle progression and maturation with ovulation and fertilization.
Keywords: C.elegans/cell cycle/MAPK/meiosis/phosphatase
Introduction
The mitogen-activated protein kinase (MAPK) belongs to a family of evolutionarily conserved protein kinases that are found in all eukaryotic cells (Ferrell, 1996; English et al., 1999; Chang and Karin, 2001). MAPKs play a critical role in transducing extracellular signals within cells to control various cell fate decisions. Extracellular signals activate MAPK through the specific dual-specificity kinase MEK, which phosphorylates MAPK on critical threonine and tyrosine residues within a Thr-X-Tyr motif (Payne et al., 1991; Posada and Cooper, 1992). Several protein phosphatases that oppose the activity of MEK by dephosphorylating activated MAPK have been described (Keyse, 1995; Camps et al., 2000). In particular, the members of the dual-specificity phosphatase family such as CL100 (MKP-1), VH2 (MKP-2) or Pyst1 (MKP-3) can remove phosphate groups from both the critical threonine and tyrosine residues of activated MAPK (Alessi et al., 1993; Guan and Butch, 1995; Groom et al., 1996; Muda et al., 1996). The MAPK phosphatases (MKPs) contain a specific MAPK-binding domain located in the N-terminal half and a catalytic phosphatase domain in the C-terminal portion (Muda et al., 1998; Nichols et al., 2000). The binding of a dual-specificity MKP to an activated MAPK induces a conformational change that stimulates the catalytic MKP activity (Camps et al., 1998).
MAPK activation occurs during the maturation of oocytes in all animals (reviewed by Sagata, 1997; Nebreda and Ferby, 2000; Abrieu et al., 2001). The role of the MAPK signalling pathway during oocyte development and maturation has been studied extensively in the frog Xenopus laevis. Several studies in this system have demonstrated that the tight regulation of MAPK activity serves to coordinate oocyte maturation and cell cycle progression with ovulation and fertilization to ensure the production of diploid embryos. Fully grown but immature oocytes arrest the cell cycle at the G2/M boundary of the first meiotic division. When maturation is induced, which in Xenopus is triggered by the steroid hormone progesterone, the MOS/MEK/MAPK cascade is activated (Masui and Markert, 1971; Smith and Ecker, 1971; Nebreda et al., 1993). The MAPK signal is necessary for the efficient activation of the maturation-promoting factor (MPF), which consists of a complex formed by cyclin B and the Cdc2 kinase. MPF activation induces germinal vesicle breakdown (GVBD), and it allows the oocytes to enter the M phase of meiosis I. Recent studies have shown that MAPK signalling is not absolutely required for MPF activation in Xenopus, as oocytes can still undergo maturation when MAPK is blocked, although with a significant delay (Fisher et al., 1999; Peter et al., 2002). Moreover, in starfish and mouse oocytes, GVBD precedes MAPK activation, suggesting that in these species MAPK signalling promotes the meiosis I to II transition rather than M phase entry (Colledge, 1994; Tachibana et al., 1997).
MAPK and possibly also MPF act in a positive feedback loop on Mos that serves to amplify low signal levels and maintain high MAPK activity throughout the remaining meiotic cell cycle (Gotoh et al., 1995; Nebreda et al., 1995; Matten et al., 1996). Because of this positive feedback loop and because of the inherent ultrasensitivity of the MOS/MEK/MAPK signalling cascade (Huang and Ferrell, 1996), the activation of MAPK represents an on–off switch that evokes an all-or-none biological response in the oocytes that have received a maturation-promoting signal (Ferrell and Machleder, 1998). Two constitutively active phosphatases, a PP2A-like threonine and an unidentified tyrosine phosphatase, which inactivate MAPK at a constant rate during maturation, have been found in Xenopus oocyte extracts (Sohaskey and Ferrell, 1999). In maturing mouse oocytes, the Mos signal overcomes a phosphatase activity that inhibits MAPK signalling (Verlhac et al., 2000). However, it was unknown if specific phosphatases existed that kept MAPK in an inactive state during oocyte development to prevent the spontaneous maturation of oocytes.
In the Caenorhabditis elegans hermaphrodite, the MAPK, termed MPK-1/SUR-1 (Lackner et al., 1994; Wu and Han, 1994), is activated at two separate steps during the meiotic cell cycle, resulting in a spatially defined pattern of MPK-1 activity in the germ cells (Miller et al., 2001; Page et al., 2001). First, MPK-1 is stimulated in germ cells that are in the pachytene stage of meiotic prophase I. MPK-1 signalling in pachytene germ cells is required for the progression through pachytene and/or for the entry into diplotene/diakinesis (pachytene exit; Church et al., 1995). MPK-1 is inactivated rapidly after pachytene exit, and it remains inactive throughout diakinesis, which is the point of G2/M arrest in developing C.elegans oocytes (McCarter et al., 1999). The G2/M arrest is relieved by a maturation signal produced by the sperm that reside in a specific storage compartment termed spermatheca. The sperm secrete a major sperm cytoskeletal protein (MSP) that presumably binds to a receptor on the proximal-most oocytes to induce MPK-1 activation (Miller et al., 2001). Although a functional requirement for MPK-1 signalling during oocyte maturation has not been demonstrated, it seems likely that the MPK-1 signal promotes M phase progression and GVBD, similar to the role MAPK plays in Xenopus oocytes.
We have reported previously that the dual-specificity phosphatase LIP-1 negatively regulates MPK-1 signalling during C.elegans vulval induction (Berset et al., 2001). Moreover, we observed that total extracts from animals carrying a lip-1 loss-of-function mutation exhibited an overall increase in MPK-1 activity, suggesting that LIP-1 may inactivate MPK-1 in several additional tissues. To test this possibility, we examined whether LIP-1 inhibits MPK-1 signalling during germ cell development. In this study, we demonstrate a role for LIP-1 in establishing the spatially restricted pattern of MPK-1 activity in the hermaphrodite germline. LIP-1 is required for the inactivation of MPK-1 as germ cells exit the pachytene stage of meiotic prophase I. Maintaining MPK-1 in an inactive state after pachytene exit is necessary to allow the developing oocytes to arrest the cell cycle in diakinesis until maturation is induced by the sperm signal. Oocytes lacking LIP-1 are unable to arrest in G2/M for a prolonged time, and they enter a mitotic cell cycle without being fertilized. Thus, LIP-1 is required in the developing oocytes to coordinate cell cycle progression with ovulation and fertilization. To our knowledge, this is the first report demonstrating an in vivo function for a dual-specificity phosphatase in regulating meiotic cell cycle progression.
Results
LIP-1 inhibits MPK-1 signalling in pachytene germ cells
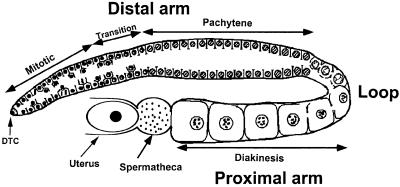
The C.elegans hermaphrodite gonad consists of two U-shaped tubes that are each connected at their proximal endings to a spermatheca where sperm are stored (Figure 1) (McCarter et al., 1999). The distal arm of each gonad forms a syncytium that contains the germ cell nuclei (Hirsh et al., 1976). In the distal-most region, the germ cells are induced by a signal from the distal tip cell to proliferate through mitotic divisions. After passing through a transition zone, germ cells enter the meiotic prophase I and progress through an extended pachytene region that occupies the remainder of the distal arm. At the loop where the distal arm turns into the proximal arm, germ cells enter diplotene/diakinesis of prophase I and arrest in diakinesis until oocyte maturation is induced. At the same time, the germ cells develop into oocytes that increase in volume and size and become cellularized while they align in a single row leading up to the spermatheca. As the oocytes approach the spermatheca, they are exposed to the MSP protein that is secreted by the sperm in the spermatheca to induce oocyte maturation, meiotic cell cycle progression and gonadal sheath cell contraction (Miller et al., 2001). The maturing oocytes enter the spermatheca where they are fertilized and then released into the uterus, a process termed ovulation (McCarter et al., 1999).
Fig. 1. Schematic diagram of a single adult hermaphrodite gonad arm. The distal gonad arm consists of a large syncytium with the circumferentially arranged nuclei on the surface and a cytoplasmic core, the central rachis (Hirsh et al., 1976). The nuclei in the distal arm are partially enclosed by plasma membranes. Each nucleus and its surrounding cytoplasm is called a germ cell (McCarter et al., 1999).
MPK-1 signalling is required for the progression of germ cells through pachytene and/or pachytene exit. In mpk-1 loss-of-function mutants, the germ cells arrest in pachytene and no oocytes develop (Church et al., 1995; Lackner and Kim, 1998). To test if the MAPK phosphatase LIP-1 inhibits MPK-1 signalling during pachytene progression, we combined mpk-1 reduction-of-function mutations (oz140 and ga111) that cause a pachytene arrest (Lackner and Kim, 1998) with the lip-1(zh15) loss-of-function mutation [termed lip-1(0) hereafter]. The mpk-1 single mutants were completely sterile when grown at the restrictive temperature, as they produced no oocytes (Table I). However, in mpk-1; lip-1(0) double mutants, oocyte development did occur and a significant fraction of the animals were fertile. Thus, LIP-1 inhibits MPK-1 signalling in pachytene germ cells.
Table I. Suppression of the mpk-1(rf) pachytene arrest phenotype by lip-1(0).
| Genotype | % sterilea | nb |
|---|---|---|
| Wild type | <1 | >100 |
| lip-1(0) | 0 | 50 |
| mpk-1(oz140)c | 100 | 50 |
| mpk-1(oz140); lip-1(0)c | 40 | 50 |
| mpk-1(ga111)c | 100 | 50 |
| mpk-1(ga111); lip-1(0)c | 4 | 50 |
aThe fertility was scored by inspecting 2- to 3-day-old adult hermaphrodites under Nomarski optics for the presence of fertilized oocytes or developing embryos.
bn indicates the number of animals observed.
cThese animals were grown at 25°C.
LIP-1 is associated with the plasma membrane of pachytene germ cells
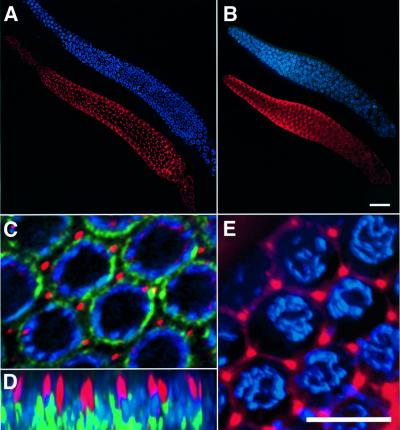
To examine the expression pattern of LIP-1 in the germline, we raised antibodies against a peptide derived from the N-terminus of LIP-1. Dissected gonads were fixed and stained with affinity-purified LIP-1 antiserum and 4′,6-diamidino-2-phenylindole di-hydrochloride (DAPI) to visualize the chromosomes. In wild-type gonads, a distinct punctate LIP-1 staining was observed in the region of the distal arm that contains pachytene nuclei (Figure 2A). In other regions of the gonad, no specific LIP-1 staining could be detected (not shown). The punctate LIP-1 staining was absent in the gonads of lip-1(0) mutants (Figure 2B). To characterize further the subcellular localization of LIP-1 in pachytene germ cells, gonads were double-stained with the LIP-1 antibody and with a nuclear pore antibody that outlined the nuclear membrane (Figure 2C and D) (Yamada and Kasamatsu, 1993). LIP-1 staining was localized between the densely packed pachytene nuclei that are arranged circumferentially near the surface of the tubular distal gonad arm. LIP-1 appeared to be concentrated in rod-like structures that measured ∼2 µm in length and extended towards the cytoplasmic core, the rachis (Figure 2D). Although the distal gonad arm is a syncytium, each germ cell nucleus is partially enclosed by a plasma membrane (Hirsh et al., 1976). When a lipophilic dye that outlines the germ cell plasma membrane was included in the staining reactions, it became apparent that LIP-1 was localized near or at the junctions where the plasma membranes that separate the different germ cells are connected to each other (Figure 2E). This specific membrane-associated localization of LIP-1 was not observed in other parts of the gonad or in somatic cells, and it may reflect a specific requirement to associate LIP-1 with individual germ cells in the gonadal syncytium in order to prevent LIP-1 from freely diffusing within the rachis. However, it is also possible that we were unable to detect by antibody staining a smaller fraction of LIP-1 that may be distributed uniformly throughout the cytoplasm, since a diffuse cytoplasmic staining may be difficult to distinguish from non-specific background staining (Figure 2B).
Fig. 2. LIP-1 expression and localization in the distal gonad arm. LIP-1 antibody staining (in red) and DAPI staining (in blue) in a dissected distal gonad arm of (A) a 2- to 3-day-old wild-type adult hermaphrodite and (B) a lip-1(0) mutant of similar age. (C) Higher magnification view of pachytene germ cells in a wild-type gonad stained with the LIP-1 antibody (in red), the nuclear pore complex antibody mAB414 (Yamada and Kasamatsu, 1993) (in green) and DAPI (in blue). (D) An xz-view of the same region as shown in (C). LIP-1 staining was concentrated in ∼2 µm long rod-like structures originating in the outer region near the pachytene nuclei and facing towards the cytoplasmic core. (E) Co-staining of LIP-1 with the hydrophobic dye Dil that labels the plasma membrane surrounding the pachytene germ cells. LIP-1 was concentrated at the junctions where membranes surrounding individual cells are connected. (A–C) Optical sections through the outer region of the distal syncytial gonad containing the germ cell nuclei. (D and E) Three-dimensional reconstructions of the entire data stacks (for details see Materials and methods). The scale bar in (B) is 20 µm and in (E) 5 µm.
LIP-1 is necessary to inactivate MPK-1 during pachytene exit
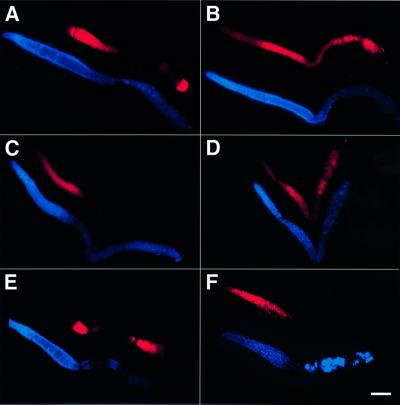
To test if loss of LIP-1 function altered the pattern of MPK-1 activation in the germline, we stained dissected gonads with a monoclonal antibody that specifically recognizes the di-phosphorylated, activated form of MPK-1 (DP-MPK-1). As previously reported (Miller et al., 2001; Page et al., 2001), MPK-1 was activated in pachytene stage germ cells and, in response to the sperm signal, in the first and sometimes second oocyte proximal to the spermatheca (Figure 3A). At other stages of germline development and after fertilization, DP-MPK-1 was barely detectable. In contrast, in the gonads of lip-1(0) mutants, a significant amount of MPK-1 remained active after pachytene exit and persisted in diakinesis oocytes in the proximal arm (Figure 3B). The increased levels of DP-MPK-1 in developing oocytes could have had two causes: LIP-1 may be required to inactivate MPK-1 during pachytene exit, or oocytes lacking LIP-1 may become hypersensitive to the MSP signal secreted by the sperm, resulting in the premature activation of MPK-1 in oocytes that are further away from the spermatheca (Page et al., 2001). To distinguish between these two possibilities, we examined the pattern of MPK-1 activation in the gonads of ‘feminized’ lip-1(0) animals that contained a temperature-sensitive mutation in the sex-determination gene fem-2 and produced no sperm when grown at the restrictive temperature (Edgar and Hirsh, 1985). In fem-2(b245ts) single mutants that lacked sperm, MPK-1 was active in pachytene cells, but no DP-MPK-1 staining was detected in the proximal-most oocytes since oocyte maturation does not take place in the absence of sperm (Figure 3C). In feminized fem-2(b245ts); lip-1(0) double mutants, however, MPK-1 remained active in developing oocytes throughout diakinesis (Figure 3D), indicating that LIP-1 is required to inactivate MPK-1 during or before pachytene exit.
Fig. 3. Activated DP-MPK-1 staining in lip-1(0) gonads. DP-MPK-1 staining (shown in red) and DAPI staining (shown in blue) in (A) wild-type, (B) lip-1(0), (C) fem-2(b245ts), (D) fem-2(b245ts); lip-1(0), (E) let-60(n1046gf) and (F) lip-(0) let-60(n1046gf) gonads. The gonads of 2- to 3-day-old adult hermaphrodites were dissected and stained with an antibody specific for the activated, di-phosphorylated form of MPK-1. The images were recorded with identical camera exposure settings, and contrast adjustments were applied simultaneously to all panels to allow a comparison of the relative signal intensities. The gonads shown in (C) and (D) are from animals that had been raised at 25°C and thus produced no sperm (Edgar and Hirsh, 1985). The gonad shown in (F) is from an animal with the full genotype lip-1(0) unc-24(e138) let-60(n1046gf); zhEx29[lip-1::gfp] that was segreg ated from a lip-1(0) unc-24(e138) let-60(n1046gf)/dpy-20(e1362) unc-22(e66); zhEx29[lip-1::gfp] mother. zhEx29 is a rescuing multicopy transgene that is expressed in most somatic cells including the somatic gonad but not in the germ cells (Berset et al., 2001). For each genotype, a minimum of 20 stained gonad arms were observed, and representative examples are shown. The scale bar in (F) is 20 µm.
To examine further the effect of LIP-1 on MPK-1 activation during germline development, we combined the lip-1(0) mutation with an activating mutation in the let-60 ras gene that is similar to the activating mutations found in human ras oncogenes (Beitel et al., 1990). The pattern of MPK-1 activation in let-60(n1046gf) single mutants was similar to that of wild-type animals, although the DP-MPK-1 staining in maturing oocytes was stronger overall, relative to wild-type oocytes (Figure 3E). In lip-1(0) let60(n1046gf) double mutants, however, high levels of DP-MPK-1 were detected throughout the distal gonad including the mitotic region (Figure 3F). Moreover, no oocytes developed in the proximal gonad arm of lip-1(0) let-60(n1046gf) animals, and the germ cells became endomitotic after pachytene exit instead of progressing to diplotene/diakinesis (see below). MPK-1 was inactivated rapidly in the endomitotic cells, similarly to the inactivation observed after fertilization (Figure 3A).
Accelerated oocyte development and partial embryonic lethality in lip-1(0) mutants
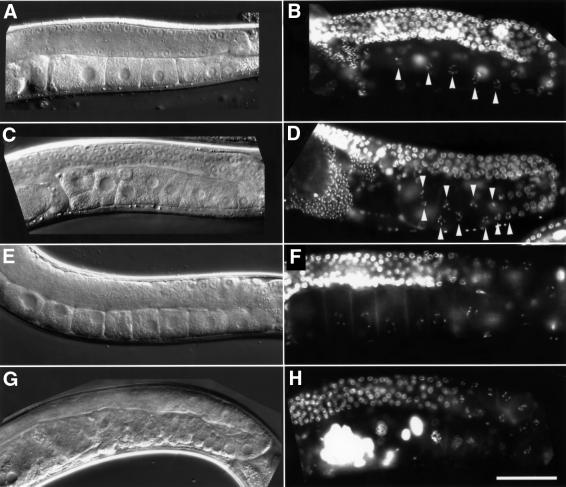
The distal gonads of lip-1(0) mutants were indistinguishable from those in wild-type animals when inspected under Nomarski optics or by DAPI staining (Figure 4A–D and data not shown). In particular, there was no apparent change in the relative numbers of the mitotic and meiotic germ cells. However, the oocytes that developed in the proximal gonads of lip-1(0) animals were often smaller than wild-type oocytes [the diameter of the smallest proximal oocytes observed in lip-1(0) mutants was approximately a quarter of the average diameter of a proximal wild-type oocyte), and they aligned in multiple rows rather than in a single row as in wild-type animals (Figure 4A–D). On average, a proximal gonad arm in a 2-day-old adult lip-1(0) animal contained ∼50% more unfertilized oocytes than the proximal gonad of a wild-type adult of similar age (Table II). One morphological feature that can be used as a marker for the timing of oocyte development is the disappearance of the nucleolus (McCarter et al., 1999; Page et al., 2001). In wild-type animals, the nucleolus disappeared in either the second (80% of the cases, n = 55) or the third oocyte (20%) prior to ovulation. In contrast, in lip-1(0) animals, the nucleolar breakdown frequently occurred prior to the third oocyte (Table II), indicating that oocyte development was accelerated in the absence of LIP-1 function. On the other hand, the rate at which oocytes were ovulated and released into the uterus was unchanged in lip-1(0) mutants (Table II). Taken together, these observations indicated that the elevated levels of activated MPK-1 caused by loss of LIP-1 function resulted in an overall acceleration of pachytene exit and oocyte development, leading to the accumulation of more, but smaller, developing oocytes in the proximal gonad arm. Two independently isolated lip-1 reduction-of-function alleles (zh31 and zh32ts; see Materials and methods) that are caused by missense mutations in the catalytic phosphatase domain exhibited a similar although less penetrant germline phenotype. It is therefore very unlikely that the phenotype observed in lip-1(0) mutants is caused by a closely linked mutation in a neighbouring gene.
Fig. 4. Accelerated oocyte development and endomitotic phenotype in lip-1(0) mutants. Nomarski micrographs of (A) a wild-type, (C) a lip-1(0), (E) a fem-2(b245ts) and (G) a fem-2(b245ts); lip-1(0) posterior gonad. DAPI-stained whole-mount preparation of (B) a wild-type, (D) a lip-1(0), (F) a fem-2(b245ts) and (H) a fem-2(b245ts); lip-1(0) animal. The arrowheads in (B) and (D) point at oocytes in diakinesis in the proximal gonad arm. Note in (C) and (D) the increased number of smaller oocytes that aligned in different planes of focus in the proximal gonad arm, and in (G) the formation of multiple endomitotic germ cells that (H) stained intensely with DAPI and often contained more than one nucleus. The animals shown in (E–H) had been raised at 25°C and thus produced no sperm (Edgar and Hirsh, 1985). The scale bar in (H) is 20 µm.
Table II. Oocyte development, maturation rates and embryonic lethality in lip-1(0) mutants and suppression by RAS/MAPK pathway mutations.
| Genotype | No. of oocytes in proximal arma (n) | % premature disappearance of the nucleolusb (n) | Maturations/hc (n) | % embryonic lethalityc (n) |
|---|---|---|---|---|
| Wild type | 7.9 ± 1.6 (46) | 0 (55) | 2.2 ± 0.2 (20) | 1.2 ± 1.3 (242) |
| lip-1(0) | 12.3 ± 4.1 (60) | 66 ± 12 (59) | 2.2 ± 0.3 (20) | 49.8 ± 4.5 (469) |
| lip-1(0) let-60(n2021) | 8.4 ± 2.5 (43) | 23 ± 14 (31) | 1.9 ± 0.5 (20) | 27.4 ± 4.7 (347) |
| lip-1(0); sem-5(n2019) | 7.9 ± 2.4 (29) | 34 ± 17 (29) | n.d. | 25.4 ± 6.4 (177) |
aThe average number of oocytes was scored by counting the number of cellularized oocytes in the proximal gonad arm of 2- to 3-day-old hermaphrodites.
bIn wild-type animals, the oocyte nucleolus disappears in either the second or the third oocyte prior to ovulation (McCarter et al., 1999; Page et al., 2001). The percentage premature nucleolar breakdown indicates the fraction of animals in which the disappearance of the nucleolus was observed before the third oocyte prior to ovulation. The disappearance of the nucleolus was observed in the fourth oocyte prior to ovulation in 29% of the lip-1(0) gonads, and in 37% before the fourth oocyte (n = 59).
cMaturation rates and embryonic lethality were scored as described in Materials and methods. Where appropriate, the 95% confidence intervals are indicated. In columns 2 and 3, (n) refers to the number of gonads examined, in column 4 to the number of animals and in column 5 to the number of embryos analysed.
About half of the oocytes that developed in lip-1(0) animals were up to 3-fold shorter along the anterior– posterior axis than wild-type oocytes. These smaller oocytes gave rise to embryos that arrested before undergoing morphogenesis. Thus, lip-1(0) mutants exhibited an incompletely penetrant maternal effect embryonic lethal phenotype (Table II). It has been reported recently that the dpl-1 and efl-1 genes negatively regulate MPK-1 activity during oocyte maturation (Page et al., 2001). The elevated levels of activated MPK-1 observed in maturing dpl-1(rf) and efl-1(rf) oocytes interfered with the subsequent establishment of the anterior–posterior polarity in the embryo, causing a Mex (muscle in excess) embryonic lethal phenotype. However, we did not observe any obvious polarity defects or a Mex phenotype in the arrested lip-1(0) embryos (see figure 7 of the Supplementary data available at The EMBO Journal Online). Thus, LIP-1 does not appear to inhibit MPK-1 signalling in the maturing oocytes.
The lip-1(0) germline phenotype is suppressed by mutations that reduce RAS/MAPK signalling activity
To test if the accelerated oocyte development and the embryonic lethality observed in lip-1(0) mutants were caused by an increased activity of the RAS/MAPK signalling pathway, we tested if mutations that partially reduce the activity of the RAS/MAPK signalling pathway suppressed the lip-1(0) germline and embryonic lethal phenotypes. For this purpose, we used reduction-of-function mutations in let-60 ras (Beitel et al., 1990) and in sem-5, which encodes an adaptor protein similar to mammalian GRB2 (Clark et al., 1992). In both lip-1(0); sem-5(n2019) and lip-1(0) let-60(n2021) double mutants, the average number of oocytes in the proximal arm, the frequency of premature nucleolar breakdown and the penetrance of the embryonic lethal phenotype were all reduced relative to lip-1(0) single mutants (Table II). Thus, a decrease in the activity of the RAS/MAPK signalling pathway partially suppressed the germline defects and the embryonic lethality caused by loss of lip-1(+) function.
Defective G2/M phase arrest in lip-1(0) oocytes
In wild-type animals, the developing oocytes in the proximal gonad arm arrest in diakinesis of meiotic prophase I until they approach the spermatheca and the secreted MSP sperm signal induces oocyte maturation and cell cycle progression (Miller et al., 2001). Oocyte nuclei in diakinesis are characterized by the presence of six bivalents that correspond to the six pairs of homologous chromosomes (Figure 5A). During oocyte maturation, the oocytes complete diakinesis and the chromosome pairs align on the metaphase plate. Since oocytes mature and ovulate every 20–30 min when sperm is present in sufficient amounts, no obvious arrest in diakinesis can be observed in wild-type animals. However, in ‘feminized’ animals that produce no sperm and therefore fail to induce oocyte maturation, the oocytes can arrest in diakinesis for several hours or even days (Figures 4E and F, and 6B) (McCarter et al., 1999). To test if lip-1(0) oocytes were capable of arresting in diakinesis, lip-1(0) animals were feminized using temperature-sensitive mutations in the sex-determination genes fem-2 (Edgar and Hirsh, 1985) or fog-1 (Schedl and Kimble, 1988). In fem-2(b245ts); lip-1(0) or fog-1(q253ts); lip-1(0) double mutants that were raised at the restrictive temperature and thus produced no sperm, oocytes frequently failed to arrest in diakinesis and became endomitotic (McCarter et al., 1997) (Figure 4G and H and Table III). The homologous chromosome pairs separated before aligning on the metaphase plate and the chromatin began to decondense (Figure 5C and D). The cells then entered a mitotic cell cycle and went through multiple rounds of DNA replication without undergoing cytokinesis or karyokinesis. As a result, highly polyploid cells with enlarged nuclei that stained intensely with DAPI accumulated in the proximal gonads of feminized lip-1(0) animals (Figure 5E and F). Moreover, 2-day-old lip-1(0) single mutants exhibited a weak endomitotic (Emo) phenotype at a low penetrance (with usually <3 endomitotic oocytes per gonad, Table III), but the Emo phenotype became stronger and more penetrant in older lip-1(0) animals that had ceased to produce sperm. To test if LIP-1 activity is required in oocytes that have arrested in diakinesis, we used the temperature-sensitive lip-1(zh32) mutation (Table IV). When oocytes were allowed to arrest at the permissive temperature (14°C) and then up-shifted to the non-permissive temperature (25°C), no significant increase in the penetrance of the Emo phenotype relative to animals that had been kept continuously at 14°C was observed (Table IV, rows 5 and 7). Moreover, the up-shifted animals that displayed an Emo phenotype usually contained fewer than three endomitotic cells per gonad arm, while the animals that had been raised at 25°C displayed a strong Emo phenotype comparable with the animal shown in Figure 4H. Taken together, these observations indicated that the inactivation of MPK-1 by LIP-1 after pachytene exit is critical for the G2/M cell cycle arrest in the developing oocytes.
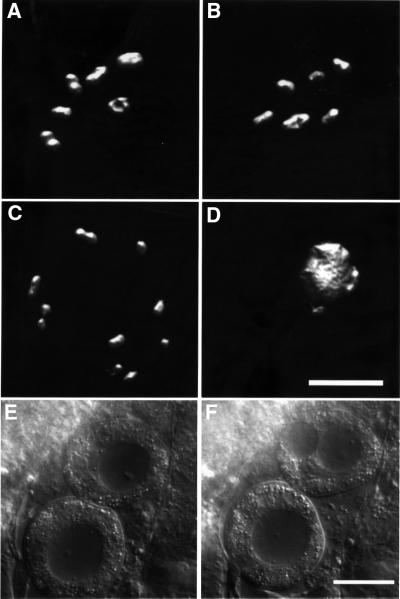
Fig. 5. Endomitotic phenotype in feminized lip-1(0) mutants. DAPI-stained condensed chromosomes in the nuclei of (A) fem-2(b245ts) and (B) lip-1(0) oocytes that have arrested in diakinesis. Both nuclei contain six bivalents that correspond to the six pairs of homologous chromosomes. (C) DAPI-stained chromosomes in the nucleus of a fem-2(b245ts); lip-1(0) oocyte that failed to arrest in diakinesis. The nucleus contains three bivalents (on the left side) and six univalent chromosomes, indicating that the homologous chromosomes have started to separate before the first meiotic division is completed. (D) A fem-2(b245ts); lip-1(0) oocyte that is undergoing endomitotic DNA replication. The decondensed chromosomes were stained intensely with DAPI. (A–D) Three-dimensional reconstructions of optical sections that were recorded and processed as described in Materials and methods. (E and F) Endomitotic oocytes in dissected fem-2(b245ts); lip-1(0) gonads were observed by Nomarski microscopy. Because of the continuous endomitotic replication, the nuclei were greatly enlarged. The nucleus in the upper oocyte started to divide, but no cytokinesis took place and karyokinesis was incomplete. The oocytes shown in (A) and (C–F) are from animals that had been raised at 25°C. The scale bars in (D) and (F) are 5 and 20 µm, respectively.
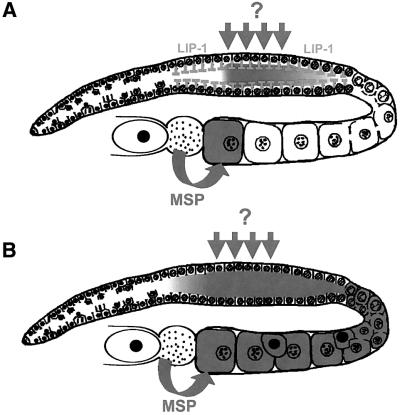
Fig. 6. A model for LIP-1 function. The dark grey color indicates the regions where MPK-1 is activated in the germline. (A) Wild-type germ cells that exit pachytene are no longer exposed to an unidentified signal (dark grey arrows) that induces MPK-1 activation during pachytene progression, but they contain LIP-1 (grey T-bars). Thus, MPK-1 is inactivated rapidly by LIP-1 during or before pachytene exit. (B) In lip-1(0) mutants, MPK-1 remains active after pachytene exit, leading to an acceleration of pachytene exit and the formation of endomitotic oocytes in the proximal gonad arm (indicated by uniformly black nuclei).
Table III. Endomitotic phenotype of unfertilized lip-1(0) oocytes.
| Genotype | % Emoa | nb |
|---|---|---|
| Wild type | 0 | 110 |
| lip-1(0) | 9 ± 5 | 122 |
| fem-2(b245ts)c | 0 | 50 |
| fem-2(b245ts); lip-1(0)c | 91 ± 5 | 108 |
| fog-1(q253ts)c | 0 | 50 |
| fog-1(q253ts); lip-1(0)c | 81 ± 9 | 68 |
| let-60(n1046gf) | 0 | 20 |
| lip-1(0) let-60(n1046gf)d | 100 | 20 |
aThe Emo phenotype was scored by examining DAPI-stained adults for the presence of intensely staining nuclei in the proximal gonad arm as described in Materials and methods. Where appropriate, the 95% confidence intervals are indicated.
bn refers to the number of animals scored.
cThese animals were raised at 25°C.
dIn addition, this strain contained the zhEx29[lip-1::gfp] transgene that rescued the somatic but not the germline phenotypes caused by loss of lip-1(+) function (Berset et al., 2001).
Table IV. Inactivation of LIP-1 in G2/M arrested oocytes.
| Row | Genotype | Temperaturea | % Emob | nc |
|---|---|---|---|---|
| 1 | Wild type | 14°C | 0 | 51 |
| 2 | Wild type | 25°C | 6 ± 8 | 35 |
| 3 | lip-1(0) | 14°C | 53 ± 23 | 17 |
| 4 | lip-1(0) | 25°C | 100 | 25 |
| 5 | lip-1(zh32) | 14°C | 4 ± 6 | 48 |
| 6 | lip-1(zh32) | 25°C | 87 ± 9 | 55 |
| 7 | lip-1(zh32) | Up-shift to 25°C | 14 ± 8 | 70 |
aIn order to feminize the animals without using a temperature-sensitive fem mutation, hermaphrodites were grown at the indicated temperature until they had stopped producing fertilized eggs (5 days after reaching the L4 stage at 25°C and 7 days after L4 at 14°C). For the up-shift experiment shown in row 7, the animals were grown at 14°C for 7 days and then placed at 25°C for another 2 days.
bThe Emo phenotype was scored by examining adults for the presence of endomitotic oocytes in the proximal gonad arm by DAPI staining or using Nomarski optics (rows 3 and 4). Where appropriate, the 95% confidence intervals are indicated.
cn refers to the number of animals scored.
A striking defect in the meiotic cell cycle was observed in lip-1(0) let-60(n1046gf) double mutants. In these animals, only a few diplotene/diakinesis germ cells were present in the loop region and all germ cells in the proximal gonad arm had become endomitotic (Figure 3F and Table III). To test if LIP-1 acts in somatic cells or in the germ cells, we introduced into lip-1(0) let-60(n1046gf) animals a rescuing lip-1::gfp transgene that was expressed in most somatic cells including the somatic gonad but not in the germline. For this purpose, we utilized the zh29 extrachromosomal array that carries multiple copies of the lip-1::gfp transgene (Berset et al., 2001) because multicopy transgenes are usually silenced in the C.elegans germline (Kelly and Fire, 1998). The expression of LIP-1–green fluorescent protein (GFP) in the soma failed to rescue the lip-1(0) let-60(n1046gf) Emo phenotype, while the somatic lip-1(0) phenotypes such as the vulval lineage defects and the larval lethality of lip-1(0) let-60(n1046gf) double mutants were rescued efficiently by the LIP-1–GFP transgene. Thus, LIP-1 most probably acts cell-autonomously to inhibit MPK-1 signalling in the germ cells.
Discussion
MAPK activation occurs during the maturation of oocytes in all sexually reproducing animals. While much is known about the signalling pathways that stimulate MAPK during maturation, the factors that keep MAPK in an inactive state in G2/M arrested oocytes in order to prevent the spontaneous maturation of oocytes were unknown. Here, we show that the C.elegans dual-specificity phosphatase LIP-1 negatively regulates MAPK signalling in the hermaphrodite germline. LIP-1 dephosphorylates and thus inactivates MPK-1 in germ cells that exit pachytene to prevent the premature activation of MPK-1 in the developing oocytes. LIP-1 thus allows the oocytes that have not received a maturation signal to arrest the cell cycle at the G2/M boundary of meiosis I.
LIP-1 inhibits MPK-1 signalling during pachytene progression
In the hermaphrodite germline, MPK-1 is first activated in the distal gonad arm in the region containing the germ cells in pachytene (Miller et al., 2001; Page et al., 2001). The LET-60 RAS/MEK-2/MPK-1 signalling cascade is absolutely required for the germ cells to progress through pachytene and enter diplotene/diakinesis (Church et al., 1995). The source and nature of the signal that induces MPK-1 signalling in the pachytene region currently are unknown. However, MPK-1 activation during pachytene progression appears to be transient, beginning in mid-pachytene and fading rapidly in late pachytene cells. Activated MPK-1 (DP-MPK-1) is virtually undetectable in germ cells that exit pachytene and enter diplotene/diakinesis. Our genetic analysis has indicated that LIP-1 negatively regulates MPK-1 signalling in pachytene germ cells, as loss of lip-1(+) function suppressed the pachytene arrest caused by reduced MPK-1 activity. Accordingly, the levels of DP-MPK-1 were elevated in the gonads of lip-1(0) animals. DP-MPK-1 staining was detected at an earlier point in pachytene lip-1(0) germ cells, and it persisted in the proximal gonad arm where the developing oocytes arrest in diakinesis. Since DP-MPK-1 staining was even present in the proximal gonad arm of lip-1(0) animals that produced no sperm, LIP-1 is probably required to inactivate MPK-1 during pachytene exit rather than to prevent the premature activation of MPK-1 by the MSP sperm signal in G2/M arrested oocytes. One model predicts that LIP-1 dephosphorylates MPK-1 at a constant rate throughout pachytene, while the signal that stimulates MPK-1 phosphorylation can only transiently override the LIP-1-mediated inhibition in the mid-pachytene region (Figure 6). Germ cells that exit pachytene may no longer be exposed to the MPK-1 stimulatory signal, but they still contain LIP-1. Thus, MPK-1 is inactivated rapidly by LIP-1 during or shortly before pachytene exit. According to this model, the failure of lip-1(0) oocytes to arrest the cell cycle in diakinesis is a consequence of the earlier loss of lip-1(+) function during pachytene progression. Consistent with this idea, LIP-1 is expressed throughout the pachytene region in the distal gonad arm. However, we cannot exclude the possibility that low levels of LIP-1 that were not detected by antibody staining may be present throughout the germline and that LIP-1 may dephosphorylate MPK-1 at additional stages of germ cell development. On the other hand, experiments with the temperature-sensitive lip-1(zh32) allele have suggested that LIP-1 activity is not required in the oocytes once they have arrested in G2/M.
In contrast to its uniform subcellular localization in somatic cells (Berset et al., 2001), LIP-1 is localized at or near the junctions between the plasma membranes that partially enclose the individual germ cells in the distal gonadal syncytium (Hirsh et al., 1976). What could be the reason for this specific membrane-associated localization of LIP-1 in pachytene germ cells? One possible explanation is that LIP-1 may be associated with the plasma membrane of germ cells to prevent LIP-1 from freely diffusing within the gonadal syncytium. LIP-1 may need to remain localized and concentrated in the pachytene germ cells where MPK-1 is activated in order to dephosphorylate MPK-1 efficiently. The DP-MPK-1 staining in the pachytene region was most intense in the cytoplasmic core of the gonadal syncytium, and no specific staining could be observed in the pachytene nuclei in wild-type or lip-1(0) germ cells. In maturing oocytes, on the other hand, DP-MPK-1 was translocated efficiently into the nuclei (Miller et al., 2001; Page et al., 2001). It thus appears that activated MPK-1 is excluded specifically from the nuclei of pachytene germ cells independently of LIP-1 function.
Loss of LIP-1 function causes accelerated oocyte development and embryonic lethality
It was unknown whether MPK-1 signalling constitutes a rate-limiting step that determines the speed of pachytene progression. The lip-1(0) mutation allowed us to examine the consequences of MPK-1 hyperactivation in the germline. Germ cells lacking LIP-1 exit pachytene at an accelerated rate, suggesting that MPK-1 signalling is indeed a rate-limiting factor during pachytene progression. Since the maturation rate (the rate at which oocytes are fertilized and released into the uterus) is unchanged in lip-1(0) mutants, more diakinesis stage oocytes accumulate in the proximal gonad than in wild-type animals. The smaller size of the lip-1(0) oocytes that develop in the proximal gonad is probably a consequence of the accelerated pachytene exit. The formation of cellularized oocytes begins in the loop that connects the distal gonadal syncytium with the proximal gonad arm where germ cells exit pachytene (McCarter et al., 1999). Since in lip-1(0) mutants a constant volume of cytoplasm is distributed among an increased number of germ cells that exit pachytene, the oocytes that develop in the proximal gonads are on average smaller than wild-type oocytes. The small lip-1(0) oocytes arrest before embryonic morphogenesis takes place, but they do not exhibit obvious polarity or patterning defects. Due to their smaller volume, the lip-1(0) oocytes may incorporate less maternal gene products, causing the embryos to arrest because they run out of multiple essential factors required for embryogenesis.
LIP-1 prevents spontaneous oocyte maturation
Experiments with Xenopus oocytes have shown that the response of the oocytes to the maturation signal has the characteristics of an all-or-none cell fate switch (Ferrell and Machleder, 1998). Immature, G2/M arrested oocytes exhibit very low MAPK activity, while maturing oocytes display almost complete MAPK activation. The MAPK signal is sufficient but not absolutely required for M phase entry (Fisher et al., 1999; Peter et al., 2002).
In C.elegans germ cells, the inactivation of MPK-1 by LIP-1 before or during pachytene exit is critical to keep MPK-1 activity at a low level during diakinesis in order to prevent the spontaneous activation of MPK-1 in the absence of a maturation signal. By analogy to Xenopus, the high levels of activated MPK-1 in lip-1(0) oocytes are most probably responsible for the failure to arrest at the G2/M boundary of meiotic prophase I. Instead, the oocytes undergo multiple rounds of mitotic divisions without cytokinesis, leading to the formation of highly polyploid endomitotic cells in the proximal gonad arm (Iwasaki et al., 1996; McCarter et al., 1997). It is unclear how far the lip-1(0) oocytes progress in the meiotic cell cycle before they enter the mitotic cell cycle. However, the absence of an ordered chromosome segregation suggests that the endomitotic lip-1(0) oocytes have entered mitosis before undergoing a reductional first meiotic division. It thus appears that in C.elegans, MPK-1 activation is not sufficient to promote the completion of meiosis I. Normal oocyte maturation and cell cycle progression may require ovulation to occur simultaneously, as mutations that block ovulation and thus trap the maturing oocytes in the proximal gonad arm result in an endomitotic phenotype (Clandinin et al., 1998).
The function of LIP-1 in establishing the fate switch from a G2/M arrested to a maturing oocyte is reminiscent of the role LIP-1 plays during vulval cell fate specification (Berset et al., 2001). During vulval induction, the central vulval precursor cell P6.p adopts a primary cell fate in response to an inductive signal that activates the MAPK cascade. The neighbouring vulval precursor cells (P5.p and P7.p) that receive slightly less inductive signal adopt the secondary cell fate because they receive a lateral Notch signal that induces LIP-1 expression to prevent the induction of the primary cell fate. In both cases, during oocyte maturation and in the primary versus secondary cell fate decision in the vulval precursor cells, LIP-1 is used to build a cell fate switch that translates a diffusible extracellular signal into an all-or-none response.
Materials and methods
General methods and strains
Caenorhabditis elegans strains were cultured at 20°C and manipulated as described (Brenner, 1974). Wild-type refers to C.elegans variety Bristol, strain N2. Unless noted otherwise, the mutations used are described in Riddle et al. (1997), and are listed below. LGI: fog-1(q253ts); LGIII: mpk-1(ga111ts) (Lackner and Kim, 1998), mpk-1(oz140ts) (Lackner and Kim, 1998), dpy-17(e164), unc-79(e1068), fem-2(b245ts); LGIV: let-60(n2021), let-60(n1046gf), unc-24(e138), lip-1(zh15) (Berset et al., 2001) [zh15 is a lip-1 null allele that is referred to as lip-1(0)], lip-1(zh31), lip-1(zh32) [The zh31 and zh32 alleles were isolated independently in a genetic screen for mutations that cause vulval patterning defects (T.Berset and A.Hajnal, unpublished results). The zh31 mutation changes Val265 to methionine and the zh32 mutation changes Pro306 to leucine. The zh32 mutation is temperature-sensitive as it causes 9% lethality at 14°C (n = 96) and 67% lethality at 25°C (n = 180)], dpy-20(e1362), unc-22(e66); LGX: sem-5(n2019); extrachromosomal array: zhEx29[lip-1::gfp] (Berset et al., 2001).
Construction of double mutants
To identify lip-1(zh15) homozygous animals, a three primer PCR assay as described in the supplementary data of Berset et al. (2001) was used, or the germline phenotype was scored by observation under Nomarski optics as described below. To identify mpk-1 homozygous strains, the mpk-1(ga111) and mpk-1(oz140) mutations were cis-linked to the unc-79(e1068) and dpy-17(e164) mutations, respectively. Homozygous fem-2(b245ts) and fog-1(q253ts) mutants were identified by placing >20 F1 progeny animals that were segregated by putative fem-2 or fog-1 homozygous mothers at 25°C and scoring them for the absence of fertilized oocyte production and the absence of viable F2 progeny. The lip-1(zh15) let-60(n1046gf) double mutant was cis-linked with the unc-24(e138) mutation and balanced over dpy-20(e1362) unc-22(e66). Homozygous lip-1(zh15) let-60(n1046gf) animals were identified by picking the Unc non-Dpy animals.
Analysis of the lip-1(0) germline phenotypes
For all experiments, 2- to 3-day-old hermaphrodites were examined unless noted otherwise. The fertility, the average number of cellularized oocytes in the proximal gonad arm and the disappearance of the nucleolus were all scored using Nomarski optics as described (Brenner, 1974). The maturation rates were determined by counting the number of fertilized oocytes that were produced in a 4 h time interval as described (McCarter et al., 1999). To quantify the embryonic lethality of lip-1 single and lip-1; sem-5 or lip-1 let-60 double mutants, a known number of embryos were transferred to unseeded NGM plates and the number of hatched larvae was counted 16–24 h later. The endomitotic (Emo) phenotype was scored by DAPI staining whole adults that had been fixed for 45 min in 4% paraformaldehyde/phosphate-buffered saline (PBS) solution and permeablized for 5 min with methanol. Percentage Emo refers to the fraction of animals that contained intensely staining germ cell nuclei with decondensed chromatin in the proximal gonad.
Production of LIP-1 antibodies
To produce LIP-1 antisera, a 20mer peptide with the sequence HLPSTSQNGEEISAEQFNRI corresponding to residues 4–23 in the LIP-1 peptide sequence was synthesized and coupled to 8-MAP carrier beads (Research Genetics Inc.). New Zealand White rabbits were injected with 0.5 mg of the antigen in complete Freund’s adjuvant and boosted three times at 4 week intervals with 0.5 mg of the antigen in incomplete Freund’s adjuvant. The anti-peptide antibody titre was followed with an enzyme-linked immunosorbent assay (ELISA). To affinity purify the LIP-1 antiserum, 2 mg of the LIP-1 20mer peptide were coupled to an NHS-activated Hi-trap column and the LIP-1 antiserum was purified according to the manufacturer’s protocol (Pharmacia). The affinity-purified LIP-1 antibodies were pre-adsorbed for 12–16 h to methanol-fixed lip-1(zh15) animals and the supernatant was used at a dilution of 1:200.
Immunofluorescence and microscopy
Adult gonads were dissected and fixed as described (Page et al., 2001). The samples were incubated with the indicated primary antibodies diluted in PBS, 0.05% Triton X-100 and 3% bovine serum albumin (BSA) for 12–16 h at room temperature, washed three times for 20 min and incubated with the appropriate tetramethylrhodamine isothiocyanate (TRITC)- or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson Laboratories) before mounting in 50% Mowiol solution. To stain the nuclear DNA, 1 µg/ml DAPI was included in all secondary antibody stainings. For Figure 2, a mouse monoclonal anti-nuclear pore antibody (mAB414, Abcam) was used at a dilution of 1:1000 to outline the nuclear membrane. To stain the plasma membrane of the germ cells, the hydrophobic dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes D-282) was included at a dilution of 1:1000. For the detection of the activated form of MPK-1, a mouse monoclonal antibody that specifically binds to the di-phosphorylated form of MAPK from different species including C.elegans (Miller et al., 2001; Page et al., 2001) was used at a dilution of 1:200 (Sigma, M8159). Pictures were taken with a Leica DMRA wide-field microscope equipped with a Hamamatsu Orca ER cooled CCD camera and controlled by the Openlab 3.0 software package (Improvision Inc.). For the pictures shown in Figures 2 and A–D, optical z-sections were recorded and processed by 12–15 cycles of iterative deconvolution using the Openlab software package to subtract the out-of-focus light. Where indicated in the figure legends, three-dimensional reconstructions of the image stacks produced with the Volocity 1.3 software package (Improvision) are shown.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Daniel Bopp, Ernst Hafen and Fritz Müller for critical reading of the manuscript. We are also grateful to A.Puotti, G.Seydoux, S.Kim and the Caenorhabditis elegans genetics center for providing some of the strains used in this study. This research was supported by grants to A.H. from the Swiss Cancer League (Oncosuisse), the Swiss National Science Foundation and by the Kanton of Zürich.
References
- Abrieu A., Doree,M. and Fisher,D. (2001) The interplay between cyclin-B–Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes. J. Cell Sci., 114, 257–267. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Smythe,C. and Keyse,S.M. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene, 8, 2015–2020. [PubMed] [Google Scholar]
- Beitel G.J., Clark,S.G. and Horvitz,H.R. (1990) Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature, 348, 503–509. [DOI] [PubMed] [Google Scholar]
- Berset T., Hoier,E.F., Battu,G., Canevascini,S. and Hajnal,A. (2001) Notch inhibition of RAS signaling through MAP kinase phosphatase LIP-1 during C.elegans vulval development. Science, 291, 1055–1058. [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics, 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Nichols,A., Gillieron,C., Antonsson,B., Muda,M., Chabert,C., Boschert,U. and Arkinstall,S. (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science, 280, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Camps M., Nichols,A. and Arkinstall,S. (2000) Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J., 14, 6–16. [PubMed] [Google Scholar]
- Chang L. and Karin,M. (2001) Mammalian MAP kinase signalling cascades. Nature, 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Church D.L., Guan,K.L. and Lambie,E.J. (1995) Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development, 121, 2525–2535. [DOI] [PubMed] [Google Scholar]
- Clandinin T.R., DeModena,J.A. and Sternberg,P.W. (1998) Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C.elegans. Cell, 92, 523–533. [DOI] [PubMed] [Google Scholar]
- Clark S.G., Stern,M.J. and Horvitz,H.R. (1992) C.elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature, 356, 340–344. [DOI] [PubMed] [Google Scholar]
- Colledge W.H. (1994) Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature, 370, 65–68. [DOI] [PubMed] [Google Scholar]
- Edgar L.G. and Hirsh,D. (1985) Use of a psoralen-induced phenocopy to study genes controlling spermatogenesis in Caenorhabditis elegans. Dev. Biol., 111, 108–118. [DOI] [PubMed] [Google Scholar]
- English J., Pearson,G., Wilsbacher,J., Swantek,J., Karandikar,M., Xu,S. and Cobb,M.H. (1999) New insights into the control of MAP kinase pathways. Exp. Cell Res., 253, 255–270. [DOI] [PubMed] [Google Scholar]
- Ferrell J.E. Jr (1996) MAP kinases in mitogenesis and development. Curr. Top. Dev. Biol., 33, 1–60. [DOI] [PubMed] [Google Scholar]
- Ferrell J.E. Jr and Machleder,E.M. (1998) The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science, 280, 895–898. [DOI] [PubMed] [Google Scholar]
- Fisher D.L., Brassac,T., Galas,S. and Doree,M. (1999) Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development, 126, 4537–4546. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama,N., Dell,K., Shirakabe,K. and Nishida,E. (1995) Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J. Biol. Chem., 270, 25898–25904. [DOI] [PubMed] [Google Scholar]
- Groom L.A., Sneddon,A.A., Alessi,D.R., Dowd,S. and Keyse,S.M. (1996) Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J., 15, 3621–3632. [PMC free article] [PubMed] [Google Scholar]
- Guan K.L. and Butch,E. (1995) Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J. Biol. Chem., 270, 7197–7203. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim,D. and Klass,M. (1976) Development of the reproductive system of Caenorhabditis elegans. Dev. Biol., 49, 200–219. [DOI] [PubMed] [Google Scholar]
- Huang C.Y. and Ferrell,J.E.,Jr (1996) Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA, 93, 10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., McCarter,J., Francis,R. and Schedl,T. (1996) emo-1, a Caenorhabditis elegans Sec61pγ homologue, is required for oocyte development and ovulation. J. Cell Biol., 134, 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W.G. and Fire,A. (1998) Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development, 125, 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S.M. (1995) An emerging family of dual specificity MAP kinase phosphatases. Biochim. Biophys. Acta, 1265, 152–160. [DOI] [PubMed] [Google Scholar]
- Lackner M.R. and Kim,S.K. (1998) Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics, 150, 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M.R., Kornfeld,K., Miller,L.M., Horvitz,H.R. and Kim,S.K. (1994) A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev., 8, 160–173. [DOI] [PubMed] [Google Scholar]
- Masui Y. and Markert,C.L. (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool., 177, 129–145. [DOI] [PubMed] [Google Scholar]
- Matten W.T., Copeland,T.D., Ahn,N.G. and Vande Woude,G.F. (1996) Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev. Biol., 179, 485–492. [DOI] [PubMed] [Google Scholar]
- McCarter J., Bartlett,B., Dang,T. and Schedl,T. (1997) Soma–germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol., 181, 121–143. [DOI] [PubMed] [Google Scholar]
- McCarter J., Bartlett,B., Dang,T. and Schedl,T. (1999) On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol., 205, 111–128. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Nguyen,V.Q., Lee,M.H., Kosinski,M., Schedl,T., Caprioli,R.M. and Greenstein,D. (2001) A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science, 291, 2144–2147. [DOI] [PubMed] [Google Scholar]
- Muda M., Boschert,U., Dickinson,R., Martinou,J.C., Martinou,I., Camps,M., Schlegel,W. and Arkinstall,S. (1996) MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem., 271, 4319–4326. [DOI] [PubMed] [Google Scholar]
- Muda M. et al. (1998) The mitogen-activated protein kinase phosphatase-3 N-terminal noncatalytic region is responsible for tight substrate binding and enzymatic specificity. J. Biol. Chem., 273, 9323–9329. [DOI] [PubMed] [Google Scholar]
- Nebreda A.R. and Ferby,I. (2000) Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol., 12, 666–675. [DOI] [PubMed] [Google Scholar]
- Nebreda A.R., Hill,C., Gomez,N., Cohen,P. and Hunt,T. (1993) The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett., 333, 183–187. [DOI] [PubMed] [Google Scholar]
- Nebreda A.R., Gannon,J.V. and Hunt,T. (1995) Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J., 14, 5597–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. et al. (2000) Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J. Biol. Chem., 275, 24613–24621. [DOI] [PubMed] [Google Scholar]
- Page B.D., Guedes,S., Waring,D. and Priess,J.R. (2001) The C.elegans E2F- and DP-related proteins are required for embryonic asymmetry and negatively regulate Ras/MAPK signaling. Mol. Cell, 7, 451–460. [DOI] [PubMed] [Google Scholar]
- Payne D.M., Rossomando,A.J., Martino,P., Erickson,A.K., Her,J.H., Shabanowitz,J., Hunt,D.F., Weber,M.J. and Sturgill,T.W. (1991) Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J., 10, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Labbe,J.C., Doree,M. and Mandart,E. (2002) A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. Development, 129, 2129–2139. [DOI] [PubMed] [Google Scholar]
- Posada J. and Cooper,J.A. (1992) Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science, 255, 212–215. [DOI] [PubMed] [Google Scholar]
- Riddle D.L., Blumenthal,T., Meyer,B.J. and Priess,J.R. (eds) (1997) C.elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sagata N. (1997) What does Mos do in oocytes and somatic cells? BioEssays, 19, 13–21. [DOI] [PubMed] [Google Scholar]
- Schedl T. and Kimble,J. (1988) fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics, 119, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.D. and Ecker,R.E. (1971) The interaction of steroids with Rana pipiens oocytes in the induction of maturation. Dev. Biol., 25, 232–247. [DOI] [PubMed] [Google Scholar]
- Sohaskey M.L. and Ferrell,J.E.,Jr (1999) Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation in vivo. Mol. Biol. Cell, 10, 3729–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Machida,T., Nomura,Y. and Kishimoto,T. (1997) MAP kinase links the fertilization signal transduction pathway to the G1/S-phase transition in starfish eggs. EMBO J., 16, 4333–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac M.H., Lefebvre,C., Kubiak,J.Z., Umbhauer,M., Rassinier,P., Colledge,W. and Maro,B. (2000) Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBO J., 19, 6065–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. and Han,M. (1994) Suppression of activated LET-60 RAS protein defines a role of Caenorhabditis elegans SUR-1 MAP kinase in vulval differentiation. Genes Dev., 8, 147–159. [DOI] [PubMed] [Google Scholar]
- Yamada M. and Kasamatsu,H. (1993) Role of nuclear pore complex in simian virus 40 nuclear targeting. J. Virol., 67, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]