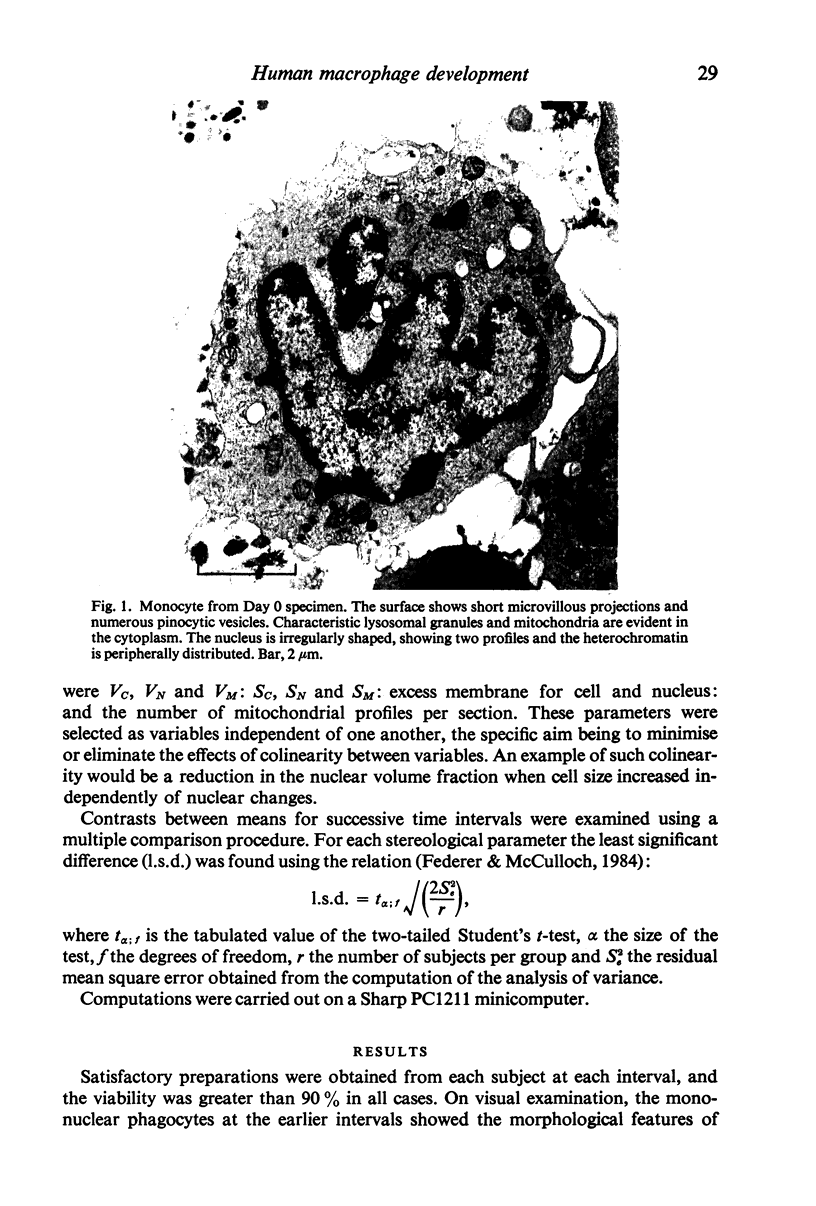
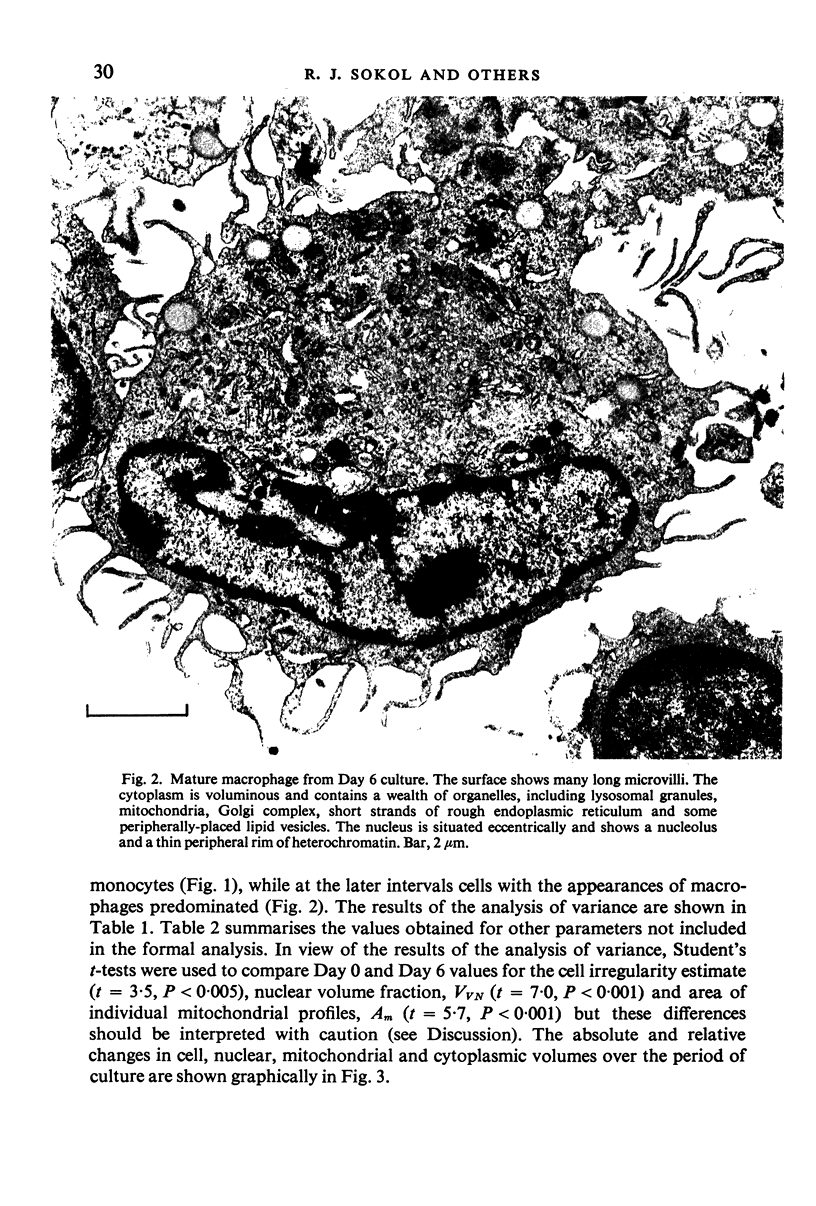
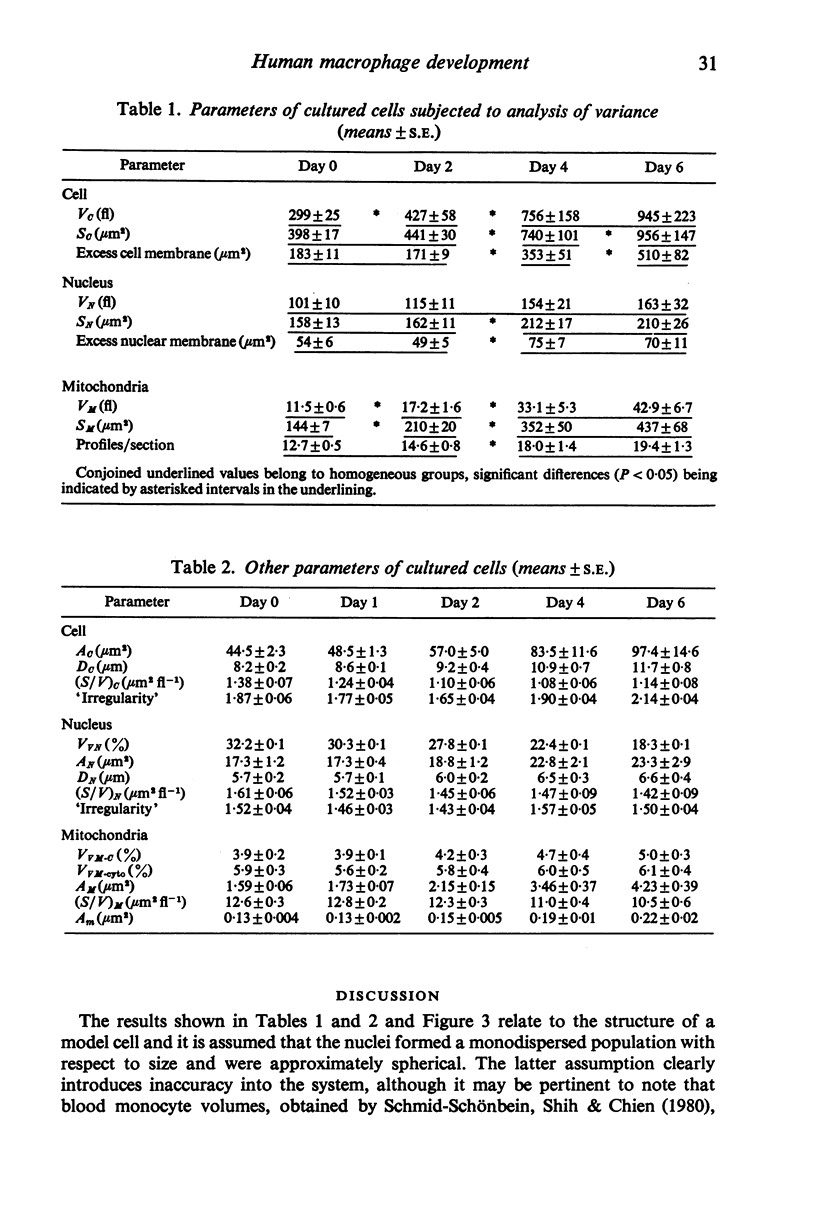
Abstract
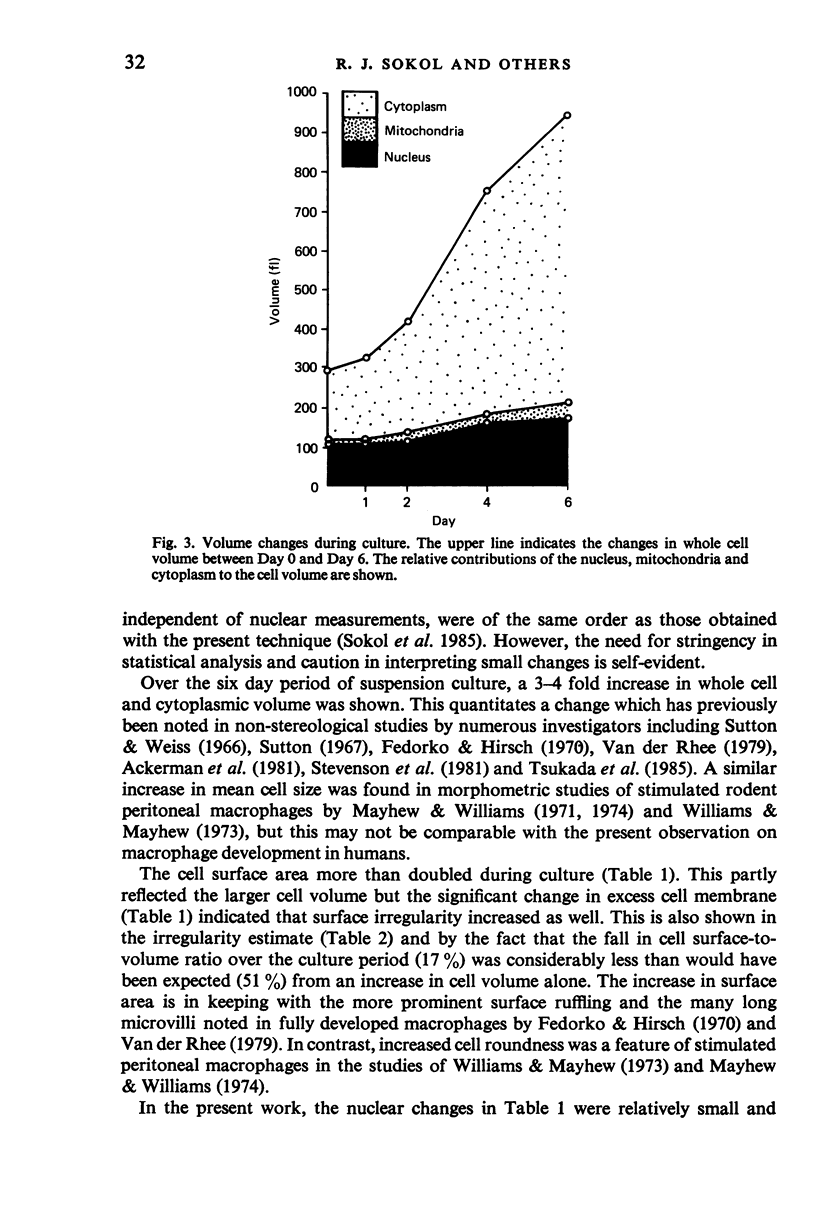
The development of macrophages from the blood monocytes of ten normal subjects has been studied at intervals over a six day period. Suspension cultures were used to obtain randomly orientated cells and morphometric measurements were made on electron micrographs. In order to meet the requirements for normality of distribution and homoscedasticity, data were logarithmically transformed. A two-way analysis of variance was then carried out, taking subjects and time intervals as fixed effects, and using a least significant difference procedure to detect variations between culture time intervals. The whole cell and cytoplasmic volumes showed 3-4 fold increases during culture. The cell surface area more than doubled; this was partly attributable to the larger cell volume and partly to increased surface irregularity. The mitochondrial volume also showed a similar significant increase, attributable to an increase in both number and size of mitochondrial profiles, the cytoplasmic volume fraction remaining approximately constant. Although there was a statistically significant increase in nuclear surface area, the nuclear changes were relatively small. The results and the application of appropriate statistical methods have thus provided basic morphometric data for human macrophage development in culture. The experimental system should permit further investigation of factors governing impaired macrophage development in malignant disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien S., Schmid-Schönbein G. W., Sung K. L., Schmalzer E. A., Skalak R. Viscoelastic properties of leukocytes. Kroc Found Ser. 1984;16:19–51. [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G. Structure of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):109–124. [PubMed] [Google Scholar]
- Mayhew T. M., Cruz L. M. Stereological correction procedures for estimating true volume proportions from biased samples. J Microsc. 1973 Dec;99(3):287–299. doi: 10.1111/j.1365-2818.1973.tb04628.x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Williams M. A. A comparison of two sampling procedures for stereological analysis of cell pellets. J Microsc. 1971 Dec;94(3):195–204. doi: 10.1111/j.1365-2818.1971.tb02369.x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Williams M. A. A quantitative morphological analysis of macrophage stimulation. I. A study of subcellular compartments and of the cell surface. Z Zellforsch Mikrosk Anat. 1974 Mar 21;147(4):567–588. doi: 10.1007/BF00307256. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Shih Y. Y., Chien S. Morphometry of human leukocytes. Blood. 1980 Nov;56(5):866–875. [PubMed] [Google Scholar]
- Sokol R. J., Durrant T. E., Hudson G. Skin window cellularity and macrophage changes in Hodgkin's and non-Hodgkin's lymphomas. Acta Haematol. 1980;64(4):209–215. doi: 10.1159/000207253. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Hudson G. Disordered function of mononuclear phagocytes in malignant disease. J Clin Pathol. 1983 Mar;36(3):316–323. doi: 10.1136/jcp.36.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R. J., Hudson G., Wales J., James N. T. Morphometry of blood monocytes in malignant lymphoma. J Clin Pathol. 1985 Aug;38(8):904–907. doi: 10.1136/jcp.38.8.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R. J., James N. T., Wales J., Hudson G. Morphometry of monocytes of human blood. Acta Anat (Basel) 1985;123(3):153–155. doi: 10.1159/000146057. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Wales J., Hudson G. Cell suspension culture for studying development of macrophages. J Clin Pathol. 1985 Oct;38(10):1194–1195. doi: 10.1136/jcp.38.10.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R. J., Wales J., Hudson G. Ultrastructure of skin window cells in malignant lymphoma. Acta Haematol. 1985;74(1):35–39. doi: 10.1159/000206161. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Wales J., Norris P. D., Hudson G. Ultrastructure of skin window cells in normal subjects. J Anat. 1982 Oct;135(Pt 3):615–624. [PMC free article] [PubMed] [Google Scholar]
- Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981 Sep;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Sutton J. S. Ultrastructural aspects of in vitro development of monocytes into macrophages, epithelioid cells, and multinucleated giant cells. Natl Cancer Inst Monogr. 1967 Sep;26:71–141. [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M., Hara Y., Sugiyama H., Yoda S., Hara T., Komiyama A., Akabane T. Quantitative evaluation of monocyte differentiation by electron microscopy: impairment of differentiation ability in monocytes from children with acute lymphoblastic leukaemia. Scand J Haematol. 1985 May;34(5):406–411. doi: 10.1111/j.1600-0609.1985.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Mayhew T. M. Quantitative microscopical studies of the mouse peritoneal macrophage following stimulation in vivo. Z Zellforsch Mikrosk Anat. 1973 Jun 28;140(2):187–202. doi: 10.1007/BF00306694. [DOI] [PubMed] [Google Scholar]
- Yoda S., Tsukada M., Hara Y., Komiyama A., Akabane T. Impaired monocyte differentiation in children with acute lymphoblastic leukaemia: its evaluation by peroxidase activity and ultrastructure. Scand J Haematol. 1984 May;32(5):525–530. doi: 10.1111/j.1600-0609.1984.tb02195.x. [DOI] [PubMed] [Google Scholar]