Abstract
Sorting nexins (SNXs) comprise a family of proteins characterized by the presence of a phox-homology domain, which mediates the association of these proteins with phosphoinositides and recruits them to specific membranes or vesicular structures within cells. Although only limited information about SNXs and their functions is available, they seem to be involved in membrane trafficking and sorting processes by directly binding to target proteins such as certain growth factor receptors. We show that SNX17 binds to the intracellular domain of some members of the low-density lipoprotein receptor (LDLR) family such as LDLR, VLDLR, ApoER2 and LDLR-related protein. SNX17 resides on distinct vesicular structures partially overlapping with endosomal compartments characterized by the presence of EEA1 and rab4. Using rhodamine-labeled LDL, it was possible to demonstrate that during endocytosis, LDL passes through SNX17-positive compartments. Functional studies on the LDLR pathway showed that SNX17 enhances the endocytosis rate of this receptor. Our results identify SNX17 as a novel adaptor protein for LDLR family members and define a novel mechanism for modulation of their endocytic activity.
Keywords: adaptor proteins/endocytosis/LDL receptor family/SNX17
Introduction
Members of the low-density lipoprotein receptor (LDLR) gene family are cell surface receptors mediating endocytosis of a variety of structurally unrelated macromolecules in many different cell types and organs. The prototype and best studied member of this family is the LDLR, which mediates the uptake of cholesterol-rich lipoproteins, thereby providing cells with cholesterol and ensuring tight systemic cholesterol homeostasis (Schneider, 1989). LDLR-related protein (LRP) binds a broad spectrum of many unrelated ligands and exerts a variety of specialized functions depending on the site of its expression (Gliemann, 1998; Krieger and Herz, 1994; Strickland et al., 1995). Megalin is an LDLR family member with structural resemblance to LRP. Although many proteins which bind to LRP are also ligands for megalin, its expression pattern and specificity for certain ligands account for physiological roles distinct from those of LRP (Moestrup et al., 1998; Nykjaer et al., 1999; Willnow, 1999).
The very low density lipoprotein receptor (VLDLR) and ApoER2 (LR7/8B) are structurally less complex than LRP and megalin, and closely resemble the LDLR (Nimpf and Schneider, 2000). LR8, the chicken homolog of the mammalian VLDLR, is well characterized. It plays a key role in yolk deposition in egg-laying species, and serves as a very instructive example for diverse functions of homologous gene products in different species (Schneider et al., 1998). The mammalian VLDLR, in concert with ApoER2, binds reelin and acts as a signaling receptor, playing a crucial role in embryonic brain development (for reviews, see Herz, 2001; Rice and Curran, 2001). The current working model proposes that ApoER2 and VLDLR directly relay the extracellular reelin signal into a cellular response via Dab1, leading to the ultimate cell responses required for the correct positioning of newly generated neurons during brain development (Howell and Herz, 2001). Since Dab1 binds not only to the intracellular domains of VLDLR and ApoER2, but also to those of LDLR and LRP (Trommsdorff et al., 1999), it is possible that signal transduction could be a function common to most members of the LDLR family. However, binding of Dab1 to members of the receptor family also seems to modulate endocytosis, as demonstrated by markedly enhanced binding of LDL to the LDLR in the presence of Dab1 (Gotthardt et al., 2000).
Mapping the locus of autosomal recessive hypercholesterolemia led to the identification of another putative intracellular adaptor protein (ARH) for the LDLR (Garcia et al., 2001). Mutations in ARH lead to a tissue-specific defect in LDLR-mediated endocytosis.
Due to a proline-rich insertion in its intracellular domain, ApoER2 binds JIP-1 and -2 linking the reelin pathway to the Jun N-terminal kinase signaling pathway (Stockinger et al., 2000). A list of further candidate binding partners for the cytoplasmic domains of members of the LDLR family (Gotthardt et al., 2000) suggests that these receptors might be part of a hitherto unknown network of events at the crossroads of endocytosis and cell signaling.
Sorting nexins (SNXs) define a family of proteins (Haft et al., 1998) involved in intracellular membrane trafficking characterized by the presence of a phox-homology (PX) domain (Ponting, 1996; Wishart et al., 2001). SNX1, the best studied mammalian member of this family, interacts with the lysosomal targeting signal of the epidermal growth factor receptor (EGFR) enhancing the rate of degradation of this surface receptor (Kurten et al., 1996). Recent mechanistic studies revealed that at least some of these proteins exert their function through their PX-domain-mediated interaction with phosphoinositol-3-phosphate [PI(3)P] (Cheever et al., 2001; Xu et al., 2001). The latest addition to the family of SNXs, i.e. SNX17, was identified recently as a potential binding partner for the cytoplasmic domain of P-selectin (Florian et al., 2001).
Here we demonstrate that SNX17 is an intracellular adaptor protein for certain members of the LDLR family, and binds to the intracellular domains of LDLR, VLDLR, ApoER2 and LRP, but not that of megalin. SNX17 resides in endosomal structures partially overlapping with EEA1- and rab4-positive structures. As exemplified by the LDLR pathway, we show that this protein participates in regulating endocytosis by enhancing the rate of receptor cycling.
Results
In order to identify proteins interacting with the cytosolic domain of ApoER2, we used the yeast two-hybrid method based on the LexA system (Mendelsohn and Brent, 1994). A Matchmaker LexA mouse brain cDNA library was screened using the intracellular domain of mouse ApoER2 lacking the 59 amino acid insert, which is subject to differential splicing (Brandes et al., 1997). Out of 108 transformants screened, 132 clones scored positive for an interaction of the receptor tail with transcripts encoded by the library. Since four of the first five clones analyzed were full-length Dab1, already identified as an adaptor for VLDLR (Trommsdorff et al., 1998; Howell et al., 1999), we designed a rapid PCR-based screening approach to identify other transcripts (see Materials and methods). Most of the clones not coding for Dab1 were either non-coding sequences interrupted by stop codons in all reading frames, or were found only once. Three overlapping clones, however, had significant homology to a human cDNA (KIAA0064) previously isolated in a random cDNA sequencing approach (Nomura et al., 1994), and these were analyzed further. The complete cDNA for this novel mouse protein was cloned by a 5′-RACE protocol using a gene-specific primer. The predicted protein has a length of 470 amino acids and contains a putative PX-domain in the N-terminus (Ponting, 1996). In a recent search for proteins interacting with the cytosolic domain of human P-selectin, KIAA0064 was identified as a potential binding partner and, based on its homology to SNXs, it was termed SNX17 (Florian et al., 2001). The novel murine adaptor protein identified in our screen exhibits 98% identity (based on protein sequence) with human SNX17, and therefore was considered to represent the murine homolog of human SNX17.
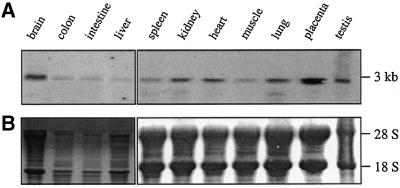
As demonstrated in Figure 1, expression of SNX17 in the mouse is very low in colon, intestine and liver. Significant expression is seen in all other major organs, with the highest levels in brain and placenta. Expression studies of the human KIAA0064 transcript, however, showed uniform expression in all tissues and cells tested, including brain and placenta (Nomura et al., 1994).
Fig. 1. Northern blot analysis of SNX17 expression in murine tissues. (A) Total RNAs (30 µg) from various tissues from male and female Balb/C mice were separated by electrophoresis on a 1.5% agarose gel and blotted as described in Materials and methods. Hybridization was performed using the full-length SNX17 probe. (B) Methylene Blue staining was used as the loading control.
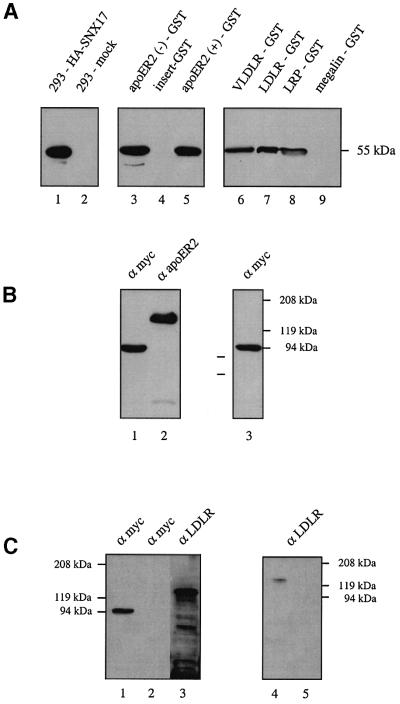
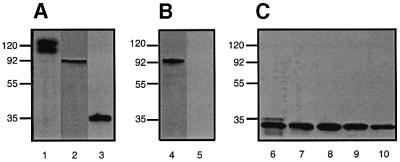
To verify the interaction between ApoER2 and SNX17 at the biochemical level, we performed ‘pull-down’ experiments. Extracts of 293 cells expressing hemagglutinin (HA)-tagged SNX17 were incubated with a bacterially expressed fusion protein between GST and the receptor tail. Protein complexes were analyzed by western blotting using a monoclonal anti-HA antibody (Figure 2A, lanes 3–5). Expression of HA-SNX17 in transfected 293 cells is demonstrated in Figure 2A (lanes 1 and 2). HA-SNX17 was indeed precipitated from cell extracts by the GST–ApoER2 tail, independently of whether or not it contains the 59 amino acid insertion (Brandes et al., 1997) (lanes 3 and 5), but not by a fusion protein of GST and the 59 amino acid insertion alone (lane 4).
Fig. 2. Interaction of SNX17 with members of the LDLR family. (A) Extracts of 293 cells expressing HA-tagged SNX17 (lane 1) or transfected with empty plasmid (lane 2) were loaded directly onto the gel, or incubated with various GST–receptor tail fusion proteins (lane 3, ApoER2 tail without the proline-rich insert; lane 4, proline-rich insert; lane 5, ApoER2 tail with the proline-rich insert; lane 6, VLDLR tail; lane 7, LDLR tail; lane 8, LRP tail; lane 9, megalin tail) pre-bound to glutathione–Sepharose for 2 h at 4°C. Bound proteins were eluted from the beads and analyzed by electrophoresis and western blotting with the anti-HA antibody. (B) Extracts of 293 cells stably expressing myc-tagged SNX17 and transiently transfected with an expression plasmid for ApoER2Δ4–6 (Brandes et al., 2001) were loaded onto the gel (lanes 1 and 2). Lane 3: an aliquot of the extract was incubated with anti-ApoER2. Immune complexes were precipitated using protein A–Sepharose, eluted from the beads and loaded onto the gel. The presence of myc-tagged SNX17 and ApoER2 was analyzed by western blotting using the indicated antibodies. (C) Extracts of MEFs grown in LPDS for 24 h and transfected with an expression plasmid for myc-tagged SNX17 (MEF-SNX17) (lanes 1 and 3) or transfected with the empty plasmid (mock; lane 2) were loaded directly onto the gel, or incubated with anti-myc antibody (9E10). Immune complexes were precipitated using protein G–Sepharose, eluted from the beads and loaded onto the gel (lane 4, MEF-SNX17; lane 5, mock). The presence of myc-SNX17 and LDLR was tested by western blotting using the indicated antibodies.
Members of the LDLR family not only share highly homologous modules in their extracellular domains, but also show high sequence similarity in their intracellular domains, including the NPxY motif important for internalization and, as recently demonstrated, also for the binding of Dab1 (Trommsdorff et al., 1998, 1999). Thus, we tested the tails of other prominent members of the receptor family for their ability to interact with SNX17. As shown in Figure 2A (lanes 6–9), SNX17 strongly associates with VLDLR, LDLR and LRP, but, surprisingly, not with megalin. In each experiment, aliquots of cell extracts corresponding to half of the number of cells from one 10 cm dish were precipitated with 10 µl of the corresponding GST–receptor tail fusion proteins. With all of the interacting receptor tails used (ApoER2, VLDLR, LDLR and LRP), precipitation of SNX17 was quantitative as determined by the absence of immunoreactivity in the cell extracts following precipitation (data not shown).
To verify these results, we performed co-immunoprecipitations using extracts from cells expressing both interacting partners. 293 cells stably expressing 9xmyc-SNX17 were transiently transfected with an expression plasmid for ApoER2Δ4–6, a variant of murine ApoER2 (Brandes et al., 2001). Simultaneous expression of both proteins was demonstrated by subjecting the cell extract to western blotting with anti-myc (9E10) and anti-ApoER2, respectively (Figure 2B, lanes 1 and 2). The cell extract was incubated with anti-ApoER2 and the immune complex was analyzed by western blotting using anti-myc antibody (9E10). As demonstrated in Figure 2B (lane 3), SNX17 is associated with ApoER2 when expressed in the same cell. This result is consistent with the ‘pull-down’ experiments shown in Figure 2A.
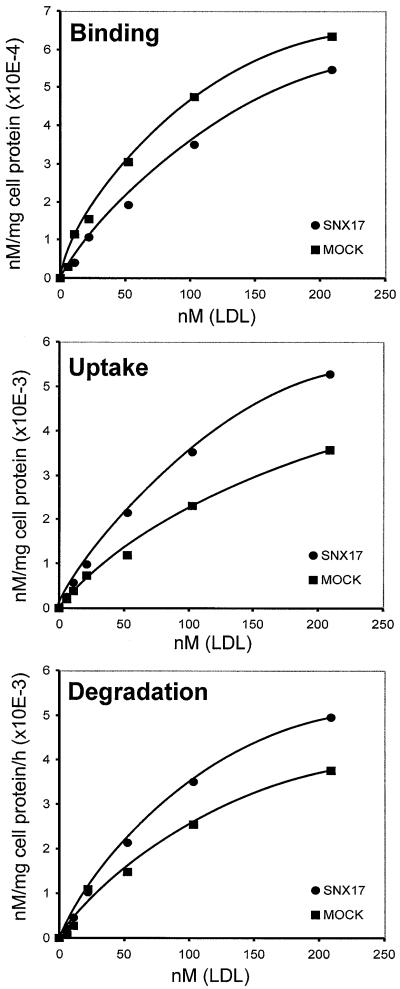
The family of SNXs is characterized by the presence of a PX-domain (Ponting, 1996; Wishart et al., 2001) and they are assumed to be involved in protein trafficking between cell organelles (Zhong et al., 2002). To test whether binding of SNX17 to members of the LDLR family interferes with receptor-mediated endocytosis, we decided to elaborate a potential function for SNX17 in the well characterized LDLR pathway. We used mouse embryo fibroblasts (MEFs) stably transfected with 9xmyc-SNX17 (MEF-SNX17) for these experiments. As seen in Figure 2C, these cells, grown in lipoprotein-deficient serum (LPDS) for 24 h prior to the experiment, express LDLR (lane 3) and 9xmyc-SNX17 at a moderate level (lane 1). To verify that SNX17 also interacts with endogenous LDLR in this cell system, co-immunoprecipitations were performed. Cell extracts derived from MEF-SNX17 cells grown in LPDS were incubated with anti-myc antibody (9E10), and the precipitate was tested for the presence of LDLR. As demonstrated in Figure 2C (lane 4), immunoprecipitation of 9xmyc-SNX17 resulted in the co-precipitation of LDLR, indicating that these proteins indeed interact in this cell system. Binding, uptake and degradation of 125I-labeled human LDL by MEF-SNX17 and a mock-transfected control cell line were then measured (Figure 3). Whereas binding of labeled LDL to SNX17-expressing cells was slightly reduced, uptake and degradation of LDL in the presence of SNX17 were significantly increased (168 and 137%, respectively, of the corresponding values in mock-transfected MEFs). To evaluate the effect of SNX17 on LDL endocytosis, we calculated the respective internalization indices. The internalization index is defined as the sum of internalized and degraded LDL divided by the amount of surface-bound LDL, and is equivalent to the number of times each LDLR cycles during a given time interval (Davis et al., 1987). The mean internalization index (calculated from the values obtained at the three highest LDL concentrations used) for MEF-SNX17 cells was approximately twice that for mock-transfected cells (18.9 versus 10.3). These results clearly demonstrate that SNX17 does not significantly influence the number of LDLRs present on the cell surface at a given time, but increases the rate of endocytosis 2-fold in the experimental set-up used. Expression of other, unrelated proteins identified in our two-hybrid screen did not interfere with LDL endocytosis.
Fig. 3. Effect of SNX17 on LDLR-mediated binding, uptake and degradation of LDL. MEFs were stably transfected with SNX17 expression plasmid or with the empty plasmid and cultivated in LPDS 24 h prior to the experiments. The monolayers were incubated with the indicated concentrations of [125I]LDL (specific activity 200 c.p.m./ng) in the absence (total binding) or presence (non-specific binding) of 500 µg/ml unlabeled LDL for 5 h at 37°C. Binding, uptake and degradation of [125I]LDL were determined as described in Materials and methods. High affinity values were calculated by subtraction of values for non-specific binding from those for total binding. Each data point represents the average of duplicate incubations.
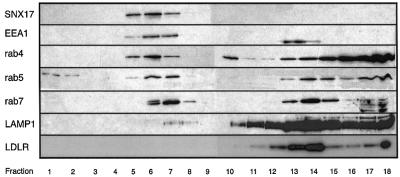
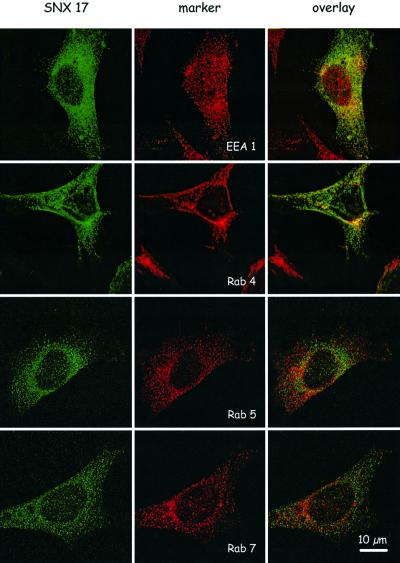
Next, we determined the intracellular localization of SNX17 in MEFs by density gradient centrifugation and immunohistochemistry. To determine the positions of relevant cellular structures, we analyzed the distribution of early endosomal antigen 1 (EEA1), rab4, rab5, rab7, LAMP1 and LDLR on a continuous 10–40% sucrose density gradient (Figure 4). SNX17 distribution overlaps with that for EEA1 and rab4; fractions showing peak expression of SNX17 are shifted towards higher densities in comparison with those for rab5 and rab7. The peak of LAMP1, a marker for the late endocytic compartment and lysosomes, localizes to heavier fractions clearly distinct from those containing SNX17. Interestingly, LAMP1 co-localizes with LDLR in the fractions with high density. These results indicate that SNX17 resides on membrane structures with a density similar to that of endosomal compartments.
Fig. 4. Analysis of the localization of SNX17 by isopycnic centrifugation on a continuous density gradient. MEFs expressing myc-tagged SNX17 were cultured in LPDS for 24 h prior to the experiment. Cells were harvested, and a post-nuclear supernatant prepared and loaded on top of a 10–40% continuous sucrose gradient as described in Materials and methods. After centrifugation, fractions were collected from the bottom of the tube and 30 µl of each fraction were subjected to electrophoresis. The respective proteins were localized by western blotting using anti-myc antibody for SNX17, and the corresponding antibodies against the respective proteins described in Materials and methods. Fractions are numbered according to increasing densities.
To refine further the structures carrying SNX17, we performed a series of co-localization experiments by immunohistochemistry. MEFs expressing myc-tagged SNX17 were stained using a monoclonal anti-myc antibody (9E10) and rabbit anti-mouse IgG conjugated to Oregon Green. As shown in Figure 5, myc-tagged SNX17 is present on small vesicular structures throughout the cytoplasm. Double staining with the antibodies against EEA1 and rab4 showed significant, but not complete, co-localization with SNX17. In comparison, there was no co-localization with rab5 and rab7, which reside on late endocytic structures on their way to lysosomes (Bucci et al., 2000). Taken together, these results demonstrate that in MEFs, SNX17 localizes to a distinct vesicle compartment which partially overlaps with the early endosomal compartment defined by the presence of EEA1 and rab4 and the absence of rab5 (Sonnichsen et al., 2000).
Fig. 5. Immunofluorescence analysis of SNX17 in MEFs and localization with reference to EEA1, rab4, rab5 and rab7. MEFs stably transfected with an expression plasmid carrying the cDNA for 9xmyc-tagged SNX17 were grown on culture slides (Becton Dickinson) and stained for SNX17 (green) and the indicated marker proteins (EEA1, rab4, rab5 and rab7; red) as described in Materials and methods. Primary antibodies were visualized with the secondary antibodies Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 594 goat anti-rabbit IgG. Bar: 10 µm.
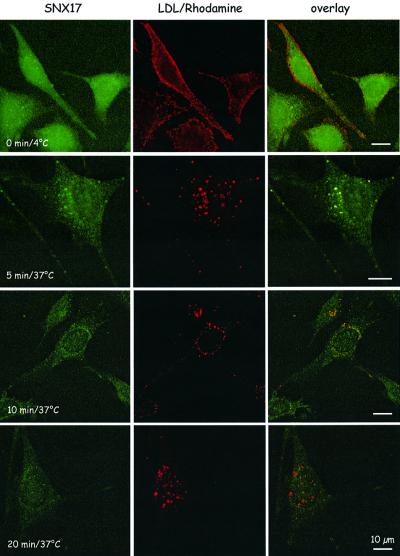
To test whether LDL endocytosed by the LDLR moves through the endosomal compartment containing SNX17, MEFs expressing myc-tagged SNX17 grown in LPDS (to induce maximal LDLR expression, cf. Figure 2C) were incubated with rhodamine-labeled LDL at 4°C to allow binding. Cells were then shifted to 37°C, and uptake of bound LDL was followed over a period of 20 min. As demonstrated in Figure 6, receptor-bound LDL does not enter the cell at 4°C. Five minutes after shifting the cells to 37°C and allowing endocytosis, LDL completely co-localizes with SNX17; at 10 min, LDL-containing compartments start to segregate from SNX17-positive vesicles, and at 20 min no co-localization of LDL and SNX17 is evident, demonstrating that during endocytosis LDL moves through an early endosomal compartment characterized by the presence of SNX17.
Fig. 6. Immunofluorescence analysis of LDL–rhodamine uptake by SNX17-expressing MEFs. MEFs stably transfected with an expression plasmid carrying the cDNA for 9xmyc-tagged SNX17 were grown in medium containing LPDS on culture slides (Becton Dickinson) and incubated with LDL–rhodamine at 4°C. Cells were then either fixed immediately (0 min) or the medium was changed and the slides were transferred to 37°C and fixed after the indicated time intervals. Cells were stained for SNX17 (green) as described in Materials and methods. Bar: 10 µm.
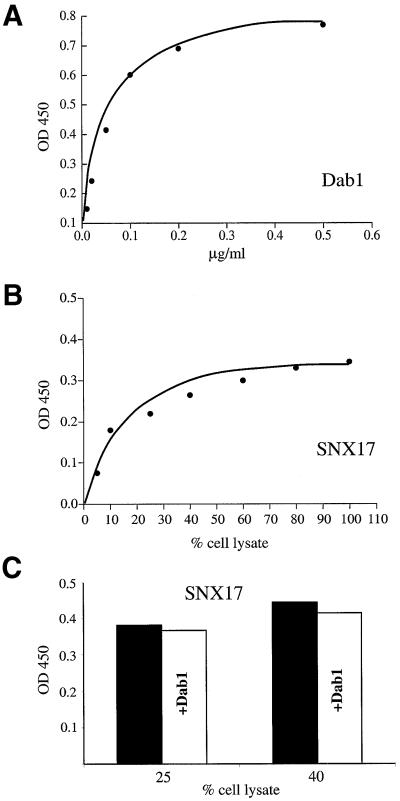
Dab1 binds to the NPxY motif present in the cytoplasmic domains of the receptors, thereby acting as an adaptor to relay the reelin signal via ApoER2 and VLDLR into migrating neurons (Trommsdorff et al., 1998). In addition, it was shown that binding of Dab1 to the LDLR interferes with receptor internalization (Gotthardt et al., 2000). To determine whether binding of SNX17 to the tails of members of the LDLR family interferes with the binding of Dab1, we developed an enzyme-linked immunosorbent assay (ELISA)-based binding method to assay the interaction of SNX17 and Dab1 with ApoER2. As demonstrated in Figure 7A, binding of Dab1 to the receptor tail is saturated at a concentration of ∼0.2 µg/ml. Binding of SNX17 to the receptor tail is also saturable (Figure 7B), but binding parameters could not be determined, since the amount of myc-tagged ligand present in the lysate of SNX17-producing cells could not be measured. Half-maximal binding was observed when the binding reaction contained ∼20% cell lysate. Binding of SNX17 at concentrations higher than those resulting in half-maximal binding (25 and 40% of cell lysate present in the assay, see Figure 7C) was not inhibited by the addition of 2 µg/ml Dab–maltose-binding protein (MBP), which is 10 times the concentration at which Dab1 binding was saturated (Figure 7C). These experiments demonstrate that binding of SNX17 to the receptor tail is independent of Dab1 binding, and suggest that in cells expressing both adaptors, members of the LDLR family are able to bind both proteins simultaneously. To demonstrate this point directly, we performed co-immunoprecipitation experiments using 293 cells stably expressing 9xmyc-SNX17 transiently transfected with ApoER2 and Dab1-HA (Figure 8A). As demonstrated in Figure 8B, 9xmyc-SNX17 could be immunoprecipitated from cell extracts using a monoclonal antibody against the myc tag (9E10). Co-precipitation of Dab1-HA is only possible when all three proteins are expressed (Figure 8C, lane 6). Control experiments using cell extracts from mock-transfected cells (lane 7), and cells expressing Dab1-HA alone (lane 8), Dab1-HA and ApoER2 (lane 9) or Dab1-HA and SNX17 (lane 10) demonstrated that ApoER2 is necessary to co-precipitate Dab1 with SNX17, in agreement with the existence of a trimeric complex.
Fig. 7. Binding of SNX17 and Dab1 to the intracellular domain of ApoER2. Microtiter plates (96-well) coated with a GST fusion protein containing the intracellular domain of ApoER2 were incubated (A) with the indicated amounts of purified Dab1–MBP expressed in E.coli or (B) with cell extracts derived from 293T cells expressing myc-tagged SNX17. Bound Dab1–MBP was visualized with the combination of an anti-MBP antibody and HRP-conjugated goat anti-rabbit IgG. Bound myc-SNX17 was visualized using the combination of an anti-myc antibody and HRP-conjugated goat anti-mouse IgG as described in Materials and methods. The color reaction was monitored by measuring absorption at 450 nm. (C) Competition of SNX17 binding by Dab1 was measured at two selected concentrations of myc-SNX17 (corresponding to 25 and 40% of cell lysate present in the incubation medium, respectively) in the absence and presence of 2 µg/ml Dab–MBP.
Fig. 8. Interaction of SNX17 and Dab1 with ApoER2. (A) Extracts of 293 cells expressing ApoER2, 9xmyc-SNX17 and Dab1-HA were subjected to SDS–PAGE under reducing conditions and transferred to a nitrocellulose membrane. The presence of the expressed proteins was visualized by western blotting using anti-ApoER2 (lane 1), anti-myc (9E10; lane 2) and anti-HA (lane 3) antibody, respectively. Binding of the primary antibodies was visualized with HRP–goat anti-rabbit (lane 1) and HRP–goat anti-mouse (lanes 2 and 3) as described in Materials and methods. (B) Extracts of 293 cells expressing ApoER2, 9xmyc-SNX17 and Dab1-HA (lane 4) or mock-transfected cells (lane 5) were incubated with anti-myc–Sepharose. Immune complexes were eluted from the beads, and loaded onto the gel. The presence of myc-tagged SNX17 was visualized by western blotting using anti-myc antibody. (C) Extracts of 293 cells expressing ApoER2, 9xmyc-SNX17 and Dab1-HA (lane 6), mock-transfected cells (lane 7), cells expressing Dab1-HA (lane 8), cells expressing Dab1-HA and ApoER2 (lane 9) or cells expressing Dab1-HA and 9xmyc-SNX17 (lane 10) were incubated with anti-myc–Sepharose. Immune complexes were eluted from the beads, and loaded onto the gel. The presence of Dab1-HA was visualized by western blotting using anti-HA antibody and HRP-conjugated goat anti-mouse IgG as described in Materials and methods.
Discussion
The discovery that certain members of the LDLR family are involved in reelin signaling suggests that these receptors exert functions beyond endocytosis of macromolecules. In fact, they may be links between endocytosis and other cellular events by interaction with a variety of intracellular adaptor proteins. Using different receptor tails as baits in yeast-two hybrid screens, a panel of potential interacting proteins has been identified (Gotthardt et al., 2000; Stockinger et al., 2000). However, the physiological relevance of most of these interactions remains to be established. Here we have identified SNX17 as a novel adaptor protein for members of the LDLR family, and show that this protein enhances LDLR-mediated endocytosis of LDL. SNX17 belongs to a growing family of proteins that are characterized by the presence of a PX-domain. Recent studies on Vam7p in yeast (Cheever et al., 2001) and mammalian SNX3 (Xu et al., 2001) demonstrated that the PX-domain acts as a PI(3)P-specific phosphoinositide-binding module playing a key role in the subcellular localization of these proteins. SNX3 is associated with early endosomes and endosomal intermediates. Its overexpression disrupts degradation of EGF, and transport of transferrin receptor from early endosome to the perinuclear recycling compartment is inhibited by microinjection of antibodies against SNX3. SNX1, although soluble to a great extent, also associates with early endosomes, as demonstrated by co-localization with EEA1 and rab5 (Kurten et al., 2001). Moreover, SNX1 directly interacts with the EGFR, a protein that moves through the endocytic/sorting machinery of the cell (Kurten et al., 1996). Our data show that another PX-domain protein interacts with the cytoplasmic domain of certain members of the LDLR family and interferes with the rate of LDLR-mediated endocytosis of LDL. These findings further support the hypothesis that PX-domain proteins play a general role in the endocytosis and/or sorting of proteins along endosomal compartments.
The interaction of SNX17 with members of the LDLR family was identified by a yeast two-hybrid screen using the cytoplasmic domain of ApoER2. The result was confirmed by pull-down experiments and co-immunoprecipitations using two independent cell systems. Interestingly, SNX17 interacts with LDLR, VLDLR, ApoER2 and LRP, but not with megalin. This is in sharp contrast to Dab1, which also interacts with megalin (Gotthardt et al., 2000). Although the corresponding domain of megalin is not very homologous to other members of the receptor family, it contains three NPxY motifs. This fact and the result presented here that SNX17 binding is not inhibited by an excess of Dab1 indicate that the binding site for SNX17 must be distinct from the NPxY motif and, consequently, from the Dab1-binding site.
Prompted by recent findings that certain SNXs are involved in receptor sorting and/or membrane trafficking (Kurten et al., 1996; Parks et al., 2001; Xu et al., 2001), we investigated whether the interaction of SNX17 with members of the LDLR family plays a role in the endocytic pathway of these receptors, and used the well characterized LDLR pathway for these studies. MEFs, like their human counterparts, express significant levels of LDLR upon culture in lipoprotein-deficient medium. Further more, as demonstrated here, expression levels of recombinant SNX17 are moderate, so that distortion of the physiological effect, or even an opposite effect via massive overexpression of the protein as seen in 293 cells, is not expected. In this experimental setting, expression of SNX17 significantly enhances LDLR-mediated uptake and degradation of LDL without a significant change of LDL binding to the cell surface. The internalization indices show that in this cell system SNX17 increases the endocytosis rate by a factor of two, without changing the number of receptors present on the cell surface. This suggests that interaction of SNX17 with the LDLR does not influence the degradation of the receptor by directing it to the lysosome, but rather accelerates its movement through the early endocytic compartment and/or recycling along the late endosomal or perinuclear recycling compartments. This is in sharp contrast to SNX1 which directs the EGFR to the lysosome, accelerating its degradation and thus downregulating the growth factor signal transmitted by EGFR (Kurten et al., 1996). To refine this concept, we determined the localization of SNX17 in MEFs by analytical sucrose gradients and immunohistochemistry. Attempts to sediment SNX17 from cell homogenates by differential centrifugation showed that only trace amounts of the protein could be recovered from the respective pellets (data not shown). On a continuous sucrose gradient, however, a significant amount of SNX17 floats with a density similar to that of vesicular structures containing EEA1 and rab4. This is in accordance with data obtained with green fluorescent protein (GFP)-tagged SNX17 expressed in CHO cells, showing that the fusion protein is localized to vesicular structures as well as to the cytoplasm (Florian et al., 2001). Similar results have been obtained with SNX1, which was found mostly in the cytoplasm where it forms oligomers that are part of larger complexes containing additional proteins (Kurten et al., 2001). A significant portion of SNX1, however, was associated with the early and/or sorting endosomal compartments. As seen in our experiments, attempts to pellet SNX1-containing membrane fractions failed, indicating that the association of the protein with the membrane fraction is rather loose and, most probably, transient. The majority of LDLRs were found in fractions characterized by the presence of LAMP1, a marker for late endosomal compartments and lysosomes. These results indicate that SNX17 does not form a stable complex with the receptor, but rather interacts transiently with it on its way through the endocytic/recycling compartments. In fact, this assumption was confirmed by experiments (presented in Figure 6) demonstrating that endocytosed LDL particles move through SNX17-positive early endosomal compartments. The overlap with fractions containing EEA1 and rab4 was analyzed further by immunofluorescence studies. SNX17 is associated predominantly with vesicular structures, confirming the membrane association suggested by the results of the sucrose gradient analysis. Low level staining of the cytoplasm suggested that the failure to pellet SNX17 with membrane fractions by sequential centrifugation might be caused by the weak, dissociable interaction of SNX17 with the vesicular structures rather than by the actual presence of SNX17 in the cytoplasm. Furthermore, these experiments demonstrated that there is no co-localization with rab5- and rab7-containing structures. There was substantial co-localization with EEA1 and rab4, however, demonstrating that SNX17 is present on a subset of early endosomal structures that might define a distinct organelle in a mosaic of membrane domains characterized by the presence of EEA1, rab5, rab4, rab7 and rab11 (Sonnichsen et al., 2000).
SNX17 was recently identified as a candidate protein for interaction with the cytoplasmic domain of P-selectin (Florian et al., 2001). Although these results have not been confirmed at the biochemical level, they are interesting in the context of the current study. P-selectin is a cell adhesion protein transiently expressed on the surface of certain cells in order to mediate their interaction with leukocytes (McEver, 1992; McEver et al., 1995). The protein is cleared rapidly from the cell surface by endocytosis (Hattori et al., 1989) and sorting to lysosomes (Green et al., 1994). Experiments where the cytoplasmic domains of P-selectin and LDLR were exchanged to form chimeric proteins demonstrated that the intracellular domain of P-selectin is sufficient to confer rapid turnover upon the LDLR (Green et al., 1994). These results led to a model for possible sites of sorting P-selectin from the LDLR by directing P-selectin to lysosomes and LDLR to the recycling pathway, respectively. If SNX17 indeed interacts with both proteins, one would assume that this interaction could occur in compartments harboring both proteins. In this case, endocytic compartments or early endosomes are the most likely candidates, in agreement with the data presented here.
In summary, SNX17 has been identified as a novel interaction partner for members of the LDLR family. It resides in distinct parts of the early endosomal compartment and enhances the endocytosis rate of the LDLR, and possibly other surface receptors.
Materials and methods
Yeast two-hybrid screen and cDNA cloning
The cDNA coding for the cytoplasmic tail of murine ApoER2 lacking the 59 amino acid proline-rich insertion was generated as described previously (Stockinger et al., 2000). The PCR product was cloned into pLexA via the EcoRI and NotI restriction sites of the primers and used as a bait for screening a mouse brain MatchmakerR LexA yeast two-hybrid library (Clontech) according to the manufacturer’s instructions. To select for clones different from Dab1, colony PCR using Dab1-specific primers was performed (sense, 5′-GTACAAAGCCAAGCTGATTGG; antisense, 5′-CATGAACAGCATGGTGATGC). Negative colonies were selected, and the corresponding plasmid insertions were amplified selectively by colony PCR using vector-specific primers, and directly sequenced. The full-length cDNA of murine SNX17 was cloned by a 5′-RACE protocol using the gene-specific primer, 5′-GCTGGATCCTGGTCACCAAAG AGCA.
cDNA constructs
SNX17 was tagged at the 5′ end with an influenza HA- or a 9xmyc-epitope and cloned into pCIneo (Promega). cDNA constructs for respective fusion proteins of GST and the intracellular domains of VLDLR, LDLR and megalin were a kind gift of Dr J.Herz (Dallas, TX). GST fusion proteins were expressed in Escherichia coli and prepared as described previously (Stockinger et al., 1998).
Northern blot analysis
Total RNA was prepared from different tissues of a male and female Balb/C mouse using Tri Reagent (Molecular Research Center, Inc.). A 30 µg aliquot of each RNA was separated by electrophoresis on a 1.5% agarose gel and transferred onto a nylon membrane. After UV cross-linking, hybridization was performed using the partial SNX17 cDNA originally found in the yeast two-hybrid screen. Methylene Blue staining was used as quality and loading control.
Antibodies
The antibody against mouse ApoER2 was described previously (Stockinger et al., 1998). The following antibodies were obtained commercially from the sources indicated: anti-HA tag (16B12; Babco), anti-Myc (9E10, used as hybridoma supernatant from the corresponding cells; ATCC), anti-EEA1 (Affinity Bioreagents, Inc.), Oregon Green 488-labeled goat anti-mouse IgG (Molecular Probes), Alexa 594-labeled goat anti-rabbit IgG (Molecular Probes), goat anti-rabbit biotinylated IgG (Sigma), anti-MBP (E8030S; New England Biolabs), peroxidase- conjugated goat anti-mouse IgG (Jackson Immuno Research Laboratories) and peroxidase-conjugated anti-protein A (Sigma). The anti-mouse LDLR antibody was a kind gift of Dr J.Herz, Dallas, the antibodies to rab4, rab5, rab7 and Lamp1 were a kind gift of Dr L.Huber, IMP, Vienna.
Cell culture, GST pull-down assays and co-immunoprecipitations
293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) supplemented with 10% fetal calf serum (FCS) and 584 mg/l glutamine, and transiently transfected with the expression plasmid for HA-SNX17 or the empty plasmid using Lipofectin reagent (Life Technologies). Cellular extracts for western blots and precipitation experiments were prepared by lysing the cells in HUNT buffer (20 mM Tris pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.1 mg/ml phenylmethylsulfonyl fluoride) for 15 min on ice and centrifugation to remove insoluble material. For pull-down experiments, 30 µl of cell extract were diluted with 150 µl of HUNT buffer. After the addition of 10 µl of glutathione–Sepharose with bound fusion protein, the mixture was incubated for 2 h at 4°C. Subsequently, the beads were washed three times with 200 µl of HUNT buffer. Bound proteins were eluted by incubating the beads for 3 min at 95°C under reducing conditions in SDS loading buffer and subjected to SDS–PAGE. Electrophoresis and western blotting were performed as described previously (Hiesberger et al., 1995). For co-immunoprecipitation experiments, 293 cells were transiently transfected with plasmids coding for 9xmyc-SNX17 and ApoER2. The protein complex was precipitated as described above by adding 10 µl of an antiserum against ApoER2 and 10 µl of protein A–Sepharose. For the co-immunoprecipitation of SNX17 and LDLR, we generated SNX17-expressing MEFs (MEF1, obtained from Dr J.Herz). MEFs were maintained in DMEM supplemented with 10% FCS and 584 mg/l glutamine, and transfected with the expression plasmid for 9xmyc-tagged SNX17 or the empty plasmid using Lipofectin reagent. Stable transfectants were selected by the addition of 500 mg/l G418. Cells were grown in 150 mm dishes, and 48 h prior to the experiment they were switched to a medium containing LPDS. After washing with phosphate-buffered saline (PBS), the cells were lysed with 1 ml of lysis buffer (10 mM HEPES pH 6.8, 1 mM MgCl2, 0.5 mM EGTA, 8.6% sucrose, 1% Triton X-100). Insoluble material was removed by centrifugation (10 min, 1000 g, 4°C). A 300 µl aliquot of the super natant was mixed with 120 µl of hybridoma supernatant containing the anti-myc antibody (9E10) and incubated for 1 h. Immune complexes were isolated by the addition of 100 µl of a protein G–agarose suspension. The beads were washed four times with 100 µl of lysis buffer and bound proteins were eluted by incubating the beads for 3 min at 95°C under reducing conditions in SDS loading buffer, and subjected to SDS–PAGE and western blotting.
Binding, uptake and degradation assays
MEFs stably expressing SNX17 were grown in a monolayer in 60 mm dishes containing 10% FCS. Before confluency was reached, they were cultured for the last 24 h before the experiment in medium containing lipoprotein-deficient serum to induce LDLR expression. Analysis of binding, uptake and degradation of 125I-labeled human LDL was performed as described previously (Goldstein et al., 1983). The internalization index was calculated according to Davis et al. (1987).
Sucrose gradients
MEFs expressing SNX17 were cultured as described above. Confluent dishes of the cells were harvested in PBS and resuspended in 300 µl of buffer A (3 mM imidazole pH 7.4, 1 mM EDTA) containing 8.5% sucrose. The cells were disrupted by 20 strokes through a 23 gauge needle, and the efficiency was monitored by visual observation through a microscope (ID03; Zeiss). Nuclei were removed by 10 min centrifugation at 1000 g and the supernatant was loaded on top of 4 ml of a 10–40% continuous sucrose gradient (in buffer A) and spun for 16 h in a Beckmann TL-70 ultracentrifuge (swinging bucket rotor, SW60 Ti) at 40 000 r.p.m. Fractions were collected from the bottom of the tube by puncturing with a 18 gauge needle and collecting 400 µl per sample. A 30 µl aliquot of each fraction was used for western blotting.
Immunofluorescence
MEFs expressing SNX17 were grown on culture slides (Becton Dickinson) coated with poly-D-lysine to a confluency of 50–70%. After washing twice with PBS, cells were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature. After 15 min quenching with 50 mM NH4Cl, the fixed cells were incubated for 1 h with 3% goat serum, then incubated with the primary antibody for 1 h at room temperature, washed three times with PBS and incubated with the appropriate secondary antibody [Alexa Fluor 488 goat anti-mouse IgG (1:500) or Alexa Fluor 594 goat anti-rabbit IgG (1:500; Molecular Probes)]. After three washes with PBS, confocal microscopy was performed on a Leica TCS SP microscope equipped with an argon–krypton laser.
Uptake of LDL–rhodamine
Human LDL was labeled with rhodamine (Molecular Probes) according to the manufacturer’s instructions. MEFs expressing SNX17 were cultured on poly-d-lysine slides as described above. Slides were transferred on ice, washed once with PBS and incubated at 4°C for 2 h with medium containing 10 µg/ml LDL–rhodamine. Cells were then washed with PBS (4°C) and either processed immediately for fixation or medium was added and the slides were shifted for the indicated time periods to 37°C and then fixed. For fixation, cells were washed once with PBS, then treated with 4% PFA for 15 min, washed again and incubated with 50 mM NH4Cl. After blocking for 1 h with 3% goat serum in PBS, cells were incubated with the appropriate antibodies as described above. Confocal microscopy was perfomed as above.
Binding assay for SNX17 and Dab1
To measure binding affinities for the receptor–Dab1 and receptor–SNX17 interactions, we applied a solid phase binding assay using the cytosolic domain of the receptor as solid phase, and recombinant Dab1 and SNX17 as soluble ligands. Full-length Dab1 cDNA (Stockinger et al., 2000) was cloned into the pMALc-2 vector using the EcoRI and XhoI sites. The recombinant protein was expressed in E.coli, purified via an amylose resin and dialyzed against Tris-buffered saline (TBS). Full-length myc-tagged SNX17 was expressed in 293T cells, which were maintained in DMEM supplemented with 10% FCS and 584 mg/l glutamine, and transfected with Lipofectin reagent. Cellular extracts for the binding assay were prepared by lysing the cells in HUNT buffer for 15 min on ice and centrifugation to remove insoluble material. The GST fusion protein containing the intracellular domain of ApoER2 was diluted in TBS (containing 2 mM CaCl2) to a final concentration of 10 µg/ml. A 100 µl aliquot per well was used to coat microtiter plates (96-well plate) overnight at 4°C. Wells were blocked with blocking solution [TBS, 2% bovine serum albumin (BSA), 0.05% Tween, 2 mM CaCl2] for 1 h at room temperature and subsequently incubated for 1 h at room temperature with either the MBP–Dab1 fusion protein or the 293T cell extract containing the myc-tagged full-length SNX17 diluted with blocking solution. Wells were washed four times with 100 µl of blocking solution each. Bound MBP–Dab1 was visualized with an anti-MBP antibody, and bound SNX17-9xmyc using an anti-myc antibody (9E10). Wells were incubated with primary antibodies diluted in blocking solution (9E10, 1:100; anti-MBP, 1:5000) for 1 h at room temperatue, washed four times followed by a 1 h incubation with horseradish peroxidase (HRP) conjugated to the appropriate secondary antibody (goat anti-mouse, 1:40 000; goat-anti rabbit, 1:2500). Wells were washed four times with TBS before 100 µl of developing solution (100 mM sodium acetate pH 6.0, 100 µg/ml 3,3′5,5′-tetramethylbenzidine, 0.03% H2O2) were added. After 10 min, the color reaction was stopped by adding 50 µl of 1 M H2SO4. The amount of protein bound to ApoER2 was detected by measuring absorption at 450 nm.
Acknowledgments
Acknowledgements
This work was supported by Austrian Science Foundation Grants P13931-MOB and F606 (to J.N.) and F608 (to W.J.S.). We thank Egon Ogris and his group (Institute of Medical Biochemistry, University of Vienna) and Lukas Huber (IMP, Vienna) for help and advice with immunoprecipitations and immunohistochemistry.
References
- Brandes C., Novak,S., Stockinger,W., Herz,J., Schneider,W.J. and Nimpf,J. (1997) Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics, 42, 185–191. [DOI] [PubMed] [Google Scholar]
- Brandes C., Kahr,L., Stockinger,W., Hiesberger,T., Schneider,W.J. and Nimpf,J. (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not α2-macroglobulin. J. Biol. Chem., 276, 22160–22169. [DOI] [PubMed] [Google Scholar]
- Bucci C., Thomsen,P., Nicoziani,P., McCarthy,J. and van Deurs,B. (2000) Rab7: a key to lysosome biogenesis. Mol. Biol. Cell, 11, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever M.L., Sato,T.K., de Beer,T., Kutateladze,T.G., Emr,S.D. and Overduin,M. (2001) Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol., 3, 613–618. [DOI] [PubMed] [Google Scholar]
- Davis C.G., van Driel,I.R., Russell,D.W., Brown,M.S. and Goldstein,J.L. (1987) The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J. Biol. Chem., 262, 4075–4082. [PubMed] [Google Scholar]
- Florian V., Schluter,T. and Bohnensack,R. (2001) A new member of the sorting nexin family interacts with the C-terminus of P-selectin. Biochem. Biophys. Res. Commun., 281, 1045–1050. [DOI] [PubMed] [Google Scholar]
- Garcia C.K. et al. (2001) Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science, 292, 1394–1398. [DOI] [PubMed] [Google Scholar]
- Gliemann J. (1998) Receptors of the low density lipoprotein (LDL) receptor family in man. Multiple functions of the large family members via interaction with complex ligands. Biol. Chem., 379, 951–964. [PubMed] [Google Scholar]
- Goldstein J.L., Basu,S.K. and Brown,M.S. (1983) Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol., 98, 241–259. [DOI] [PubMed] [Google Scholar]
- Gotthardt M., Trommsdorff,M., Nevitt,M.F., Shelton,J., Richardson,J.A., Stockinger,W., Nimpf,J. and Herz,J. (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem., 275, 25616–25624. [DOI] [PubMed] [Google Scholar]
- Green S.A., Setiadi,H., McEver,R.P. and Kelly,R.B. (1994) The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J. Cell Biol., 124, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft C.R., Sierra,M.L., Barr,V.A., Haft,D.H. and Taylor,S.I. (1998) Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell. Biol., 18, 7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R., Hamilton,K.K., Fugate,R.D., McEver,R.P. and Sims,P.J. (1989) Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J. Biol. Chem., 264, 7768–7771. [PubMed] [Google Scholar]
- Herz J. (2001) The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron, 29, 571–581. [DOI] [PubMed] [Google Scholar]
- Hiesberger T. et al. (1995) The chicken oocyte receptor for yolk precursors as model to study the action of receptor associated protein and lactoferrin. J. Biol. Chem., 270, 18219–18226. [DOI] [PubMed] [Google Scholar]
- Howell B.W. and Herz,J. (2001) The LDL receptor gene family: signaling functions during development. Curr. Opin. Neurobiol., 11, 74–81. [DOI] [PubMed] [Google Scholar]
- Howell B.W., Lanier,L.M., Frank,R., Gertler,F.B. and Cooper,J.A. (1999) The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol., 19, 5179–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. and Herz,J. (1994) Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptor and LDL receptor-related protein (LRP). Annu. Rev. Biochem., 63, 601–637. [DOI] [PubMed] [Google Scholar]
- Kurten R.C., Cadena,D.L. and Gill,G.N. (1996) Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science, 272, 1008–1010. [DOI] [PubMed] [Google Scholar]
- Kurten R.C., Eddington,A.D., Chowdhury,P., Smith,R.D., Davidson, A.D. and Shank,B.B. (2001) Self-assembly and binding of a sorting nexin to sorting endosomes. J. Cell Sci., 114, 1743–1756. [DOI] [PubMed] [Google Scholar]
- McEver R.P. (1992) Leukocyte–endothelial cell interactions. Curr. Opin. Cell Biol., 4, 840–849. [DOI] [PubMed] [Google Scholar]
- McEver R.P., Moore,K.L. and Cummings,R.D. (1995) Leukocyte trafficking mediated by selectin–carbohydrate interactions. J. Biol. Chem., 270, 11025–11028. [DOI] [PubMed] [Google Scholar]
- Mendelsohn A.R. and Brent,R. (1994) Applications of interaction traps/two-hybrid systems to biotechnology research. Curr. Opin. Biotechnol., 5, 482–486. [DOI] [PubMed] [Google Scholar]
- Moestrup S.K. et al. (1998) The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J. Biol. Chem., 273, 5235–5242. [DOI] [PubMed] [Google Scholar]
- Nimpf J. and Schneider,W.J. (2000) From cholesterol transport to signal transduction: low density lipoprotein receptor, very low density lipoprotein receptor and apolipoprotein E receptor-2. Biochim. Biophys. Acta, 1529, 287–298. [DOI] [PubMed] [Google Scholar]
- Nomura N. et al. (1994) Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041–KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1 (supplement). DNA Res., 1, 251–262. [DOI] [PubMed] [Google Scholar]
- Nykjaer A., Dragun,D., Walther,D., Vorum,H., Jacobsen,C., Herz,J., Melsen,F., Christensen,E.I. and Willnow,T.E. (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell, 96, 507–515. [DOI] [PubMed] [Google Scholar]
- Parks W.T. et al. (2001) Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-β family of receptor serine-threonine kinases. J. Biol. Chem., 276, 19332–19339. [DOI] [PubMed] [Google Scholar]
- Ponting C.P. (1996) Novel domains in NADPH oxidase subunits, sorting nexins and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci., 5, 2353–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D.S. and Curran,T. (2001) Role of the reelin signaling pathway in central nervous development. Annu. Rev. Neurosci., 24, 1005–1039. [DOI] [PubMed] [Google Scholar]
- Schneider W.J. (1989) The low density lipoprotein receptor. Biochim. Biophys. Acta, 988, 303–317. [DOI] [PubMed] [Google Scholar]
- Schneider W.J., Osanger,A., Waclawek,M. and Nimpf,J. (1998) Oocyte growth in the chicken: receptors and more. Biol. Chem., 379, 965–971. [PubMed] [Google Scholar]
- Sonnichsen B., De Renzis,S., Nielsen,E., Rietdorf,J. and Zerial,M. (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5 and Rab11. J. Cell Biol., 149, 901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger W., Hengstschläger-Ottnad,E., Novak,S., Matus,A., Hüttinger,M., Bauer,J., Lassmann,H., Schneider,W.J. and Nimpf,J. (1998) The LDL receptor gene family: differential expression of two α2-macroglobulin receptors in the brain. J. Biol. Chem., 273, 32213–32221. [DOI] [PubMed] [Google Scholar]
- Stockinger W., Brandes,C., Fasching,D., Hermann,M., Gotthardt,M., Herz,J., Schneider,W.J. and Nimpf,J. (2000) The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J. Biol. Chem., 275, 25625–25632. [DOI] [PubMed] [Google Scholar]
- Strickland D.K., Kounnas,M.Z. and Argraves,W.S. (1995) LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J., 9, 890–898. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Borg,J.-P., Margolis,B. and Herz,J. (1998) Interaction of cytosolic adaptor proteins with neuronal apoE receptors and the amyloid presursor proteins. J. Biol. Chem., 273, 33556–33565. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Gotthardt,M., Hiesberger,T., Shelton,J., Stockinger, W., Nimpf,J., Hammer,R., Richardson,J.A. and Herz,J. (1999) Reeler/Disabled-like disruption of neuronal migartion in knock out mice lacking the VLDL receptor and apoE receptor-2. Cell, 97, 689–701. [DOI] [PubMed] [Google Scholar]
- Willnow T.E. (1999) The low-density lipoprotein receptor gene family: multiple roles in lipid metabolism. J. Mol. Med., 77, 306–315. [DOI] [PubMed] [Google Scholar]
- Wishart M.J., Taylor,G.S. and Dixon,J.E. (2001) Phoxy lipids. Revealing PX domains as phosphoinositide binding modules. Cell, 105, 817–820. [DOI] [PubMed] [Google Scholar]
- Xu Y., Hortsman,H., Seet,L., Wong,S.H. and Hong,W. (2001) SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol., 3, 658–666. [DOI] [PubMed] [Google Scholar]
- Zhong Q., Lazar,C.S., Tronchere,H., Sato,T., Meerloo,T., Yeo,M., Songyang,Z., Emr,S. and Gill,G.N. (2002) Endosomal localization and function of sorting nexin 1. Proc. Natl Acad. Sci. USA, 99, 6767–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]