Abstract
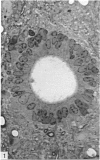
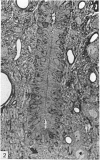
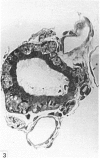
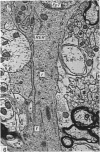
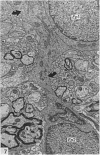
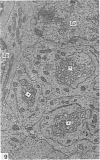
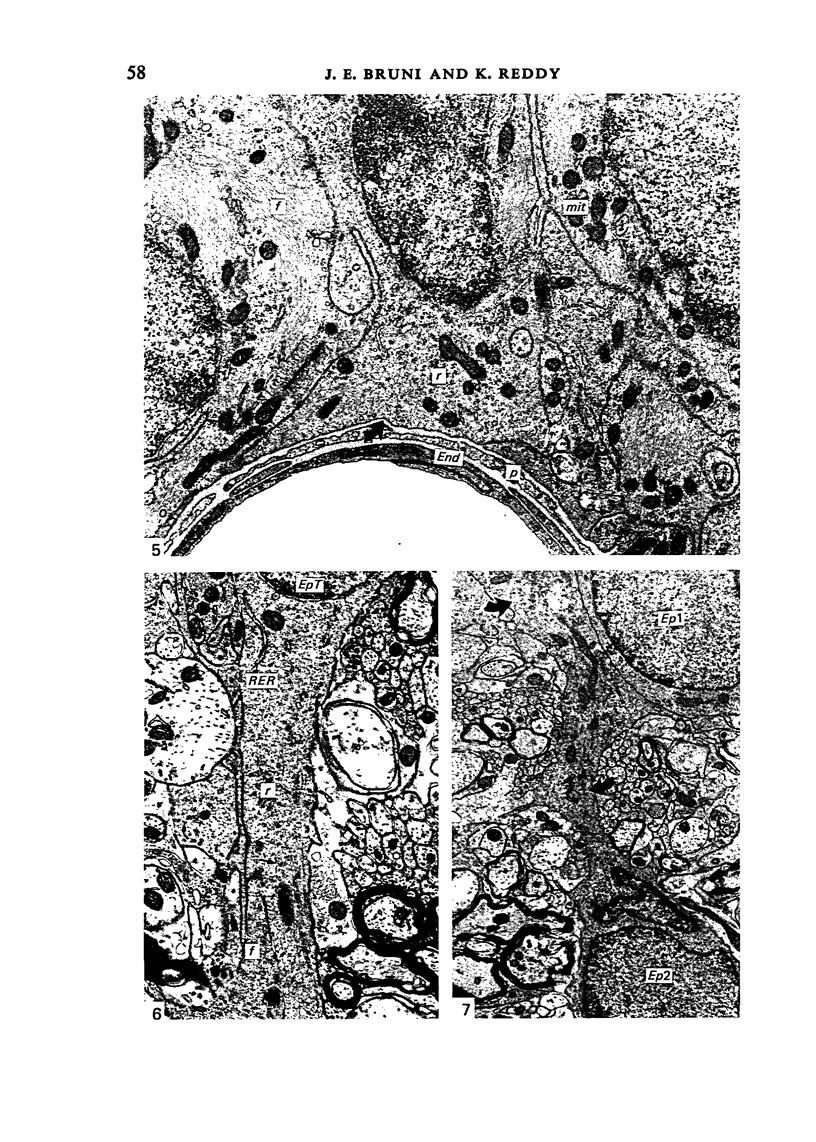
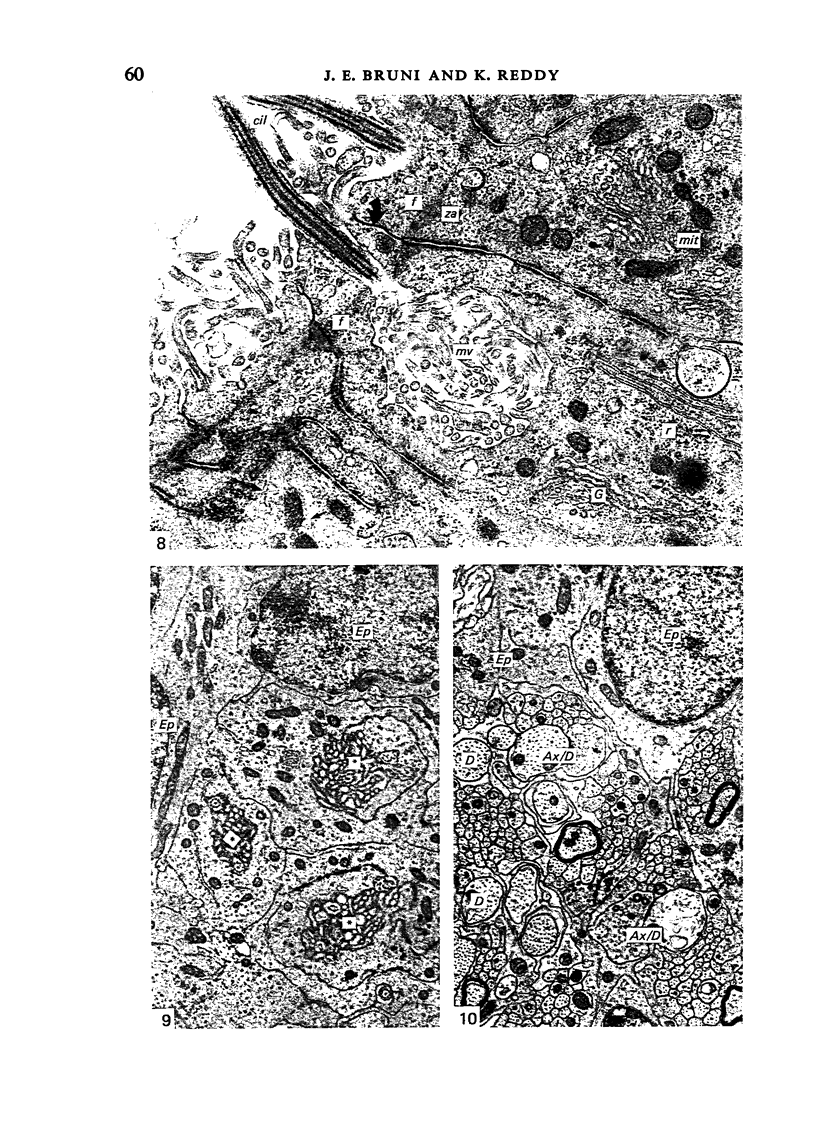
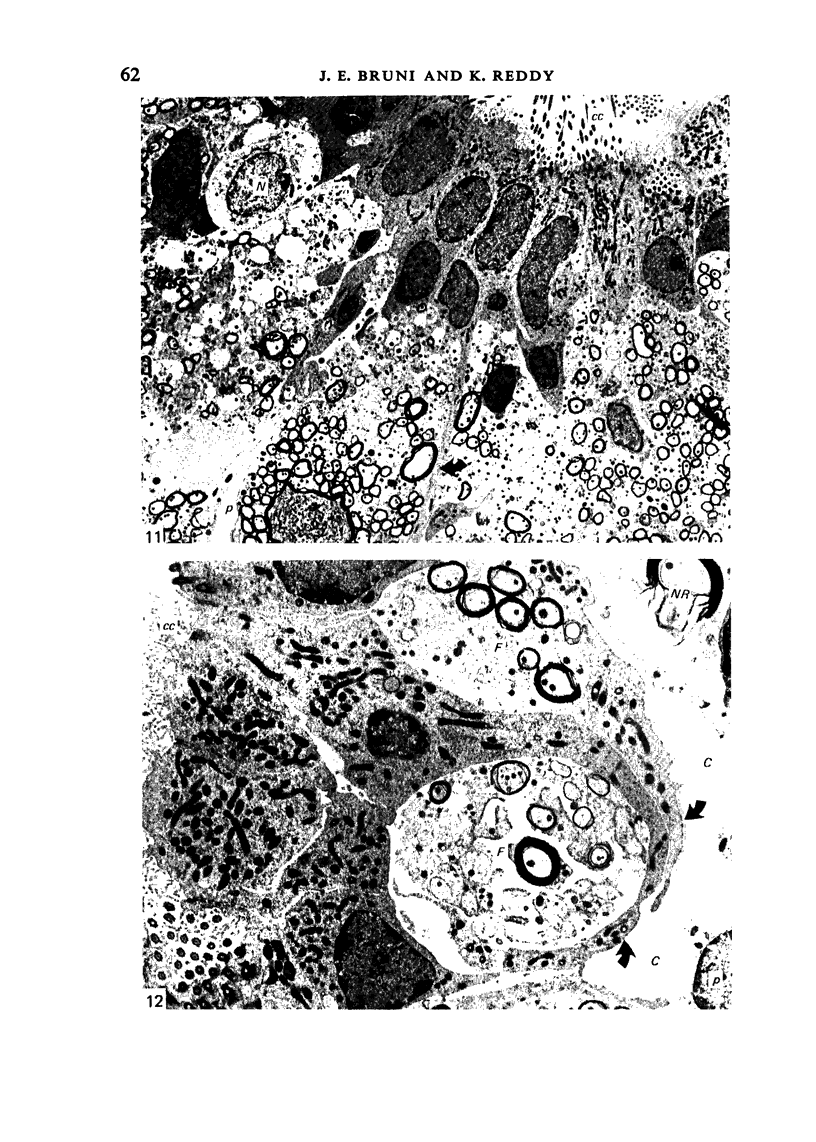
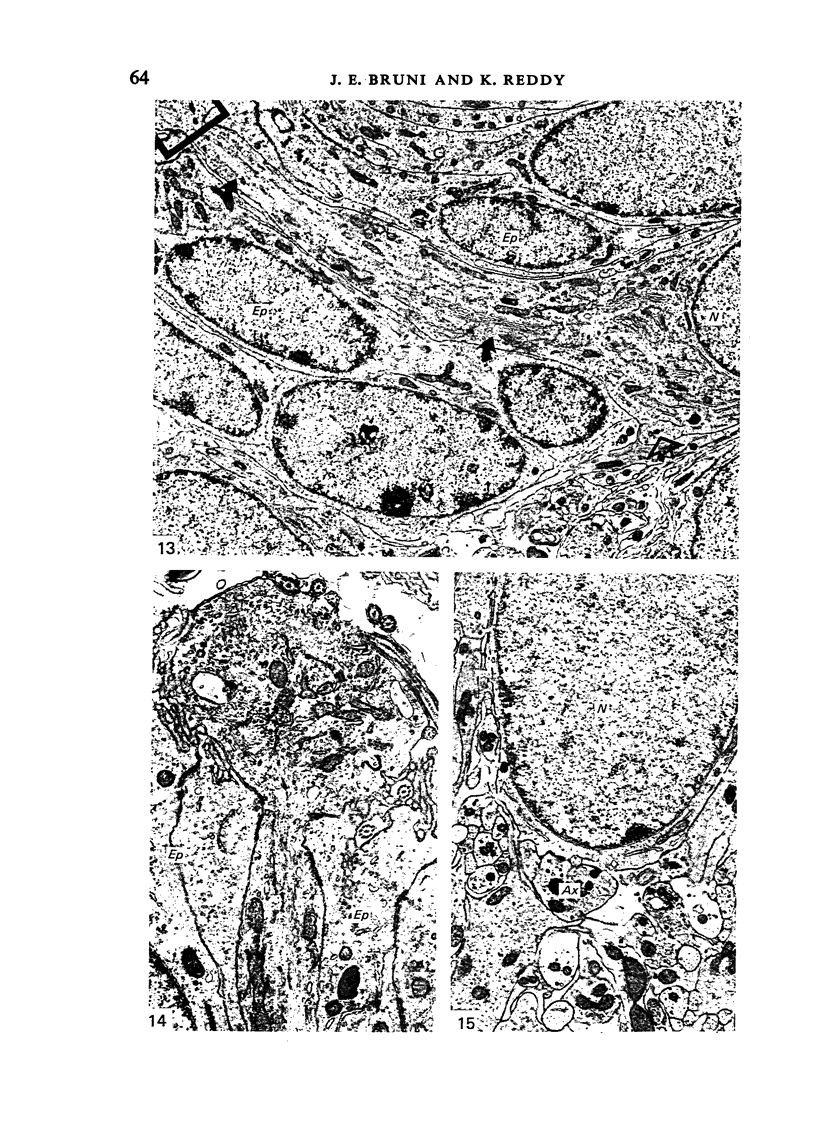
Ependymal cells of the rat central canal were examined with a view to identifying features that distinguish them regionally and from their counterparts elsewhere in the ventricular system. The results revealed that the lining consisted for the most part of a pseudostratified layer of uniformly organised cuboidal to columnar ependymal cells present in largest numbers in lumbar and sacral segments and in the conus. Two cell variants were identified on the basis of the presence or absence of a radially directed cytoplasmic process originating from the base of the cell. The tanycytic form of ependymal cell was encountered along the entire length of the central canal but with increased frequency in caudalmost segments. Ependymal cells were largely similar in ultrastructural appearance along the length of the cord. Although they were also similar in appearance and orientation to their counterparts in the ventricles they did exhibit some unique features. The most notable were the prominent junctional complexes and associated filaments present along the lateral border of the cells near their apex and the abundance of intermediate filaments in tanycytes. The central canal of the filum differed most markedly from other segments of the cord and resembled in structure the primitive ependymal tube of the caudal cord in lower vertebrates. Ependymal cells of the cord were not sufficiently dissimilar morphologically from their counterparts in the ventricles to account for differences in proliferative capacity in response to localised injury. A factor that merits further study is the difference in numbers of tanycyte ependymal cells in the two locations for they may be the reactive elements that proliferate in response to injury.
Full text
PDF

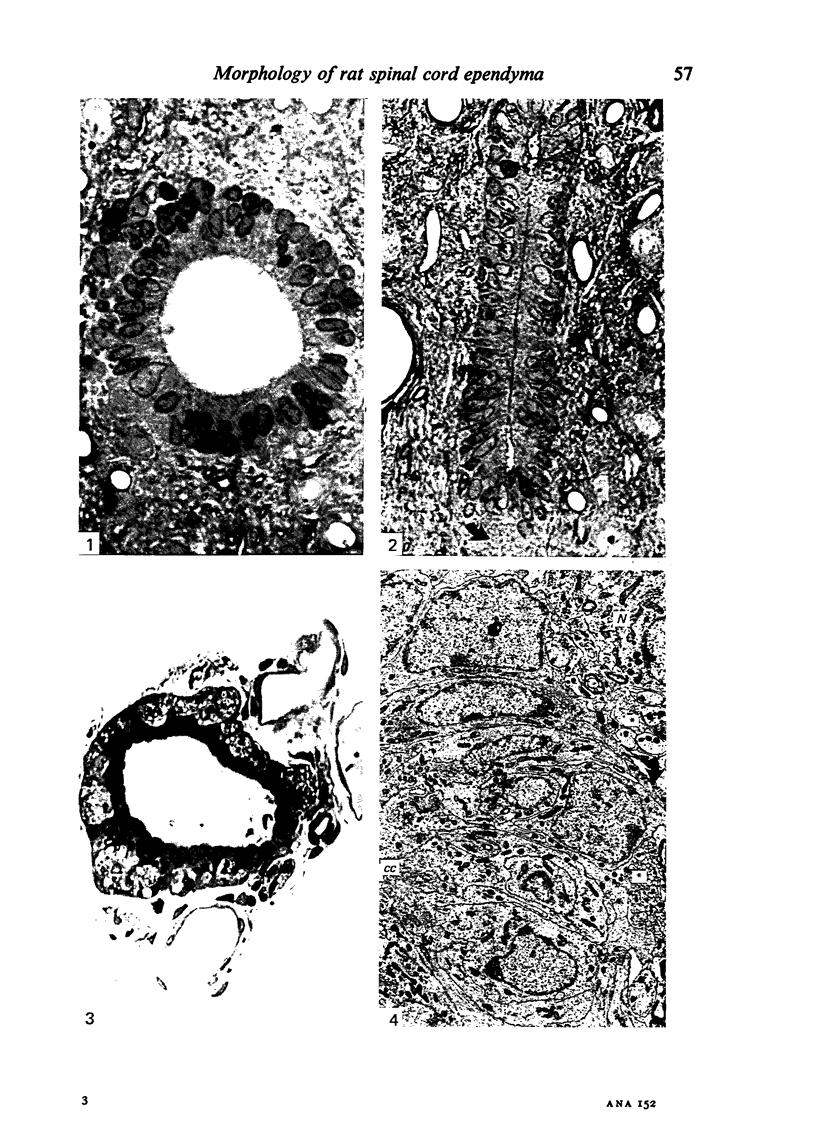









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN E. K., Jr, WALKER B. E. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962 Oct;21:597–609. doi: 10.1097/00005072-196210000-00007. [DOI] [PubMed] [Google Scholar]
- ALTMAN J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963 Apr;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Waxman S. G. Caudal spinal cord of the teleost Sternarchus albifrons resembles regenerating cord. Anat Rec. 1983 Jan;205(1):85–92. doi: 10.1002/ar.1092050111. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Waxman S. G., Laufer M. Fine structure of regenerated ependyma and spinal cord in Sternarchus albifrons. Anat Rec. 1983 Jan;205(1):73–83. doi: 10.1002/ar.1092050110. [DOI] [PubMed] [Google Scholar]
- Barber R. P., Vaughn J. E., Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Res. 1982 Apr 29;238(2):305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- Becker D. P., Wilson J. A., Watson G. W. The spinal cord central canal: response to experimental hydrocephalus and canal occlusion. J Neurosurg. 1972 Apr;36(4):416–424. doi: 10.3171/jns.1972.36.4.0416. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Lathem W. A flow of cerebrospinal fluid along the central canal of the spinal cord of the rabbit and communications between this canal and the sacral subarachnoid space. J Physiol. 1965 Dec;181(4):785–800. doi: 10.1113/jphysiol.1965.sp007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni J. E., Clattenburg R. E., Paterson J. A. Ependymal cells of the rat fourth ventricle: response to injury. Scan Electron Microsc. 1983;(Pt 2):649–661. [PubMed] [Google Scholar]
- Bruni J. E., Del Bigio M. R., Clattenburg R. E. Ependyma: normal and pathological. A review of the literature. Brain Res. 1985 Apr;356(1):1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Bryant S. V., Wozny K. J. Stimulation of limb regeneration in the lizard Xantusia vigilis by means of ependymal implants. J Exp Zool. 1974 Sep;189(3):339–352. doi: 10.1002/jez.1401890307. [DOI] [PubMed] [Google Scholar]
- Chauhan A. N., Lewis P. D. A quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain. Neuropathol Appl Neurobiol. 1979 Aug;5(4):303–309. doi: 10.1111/j.1365-2990.1979.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Chesler M., Nicholson C. Organization of the filum terminale in the frog. J Comp Neurol. 1985 Sep 22;239(4):431–444. doi: 10.1002/cne.902390409. [DOI] [PubMed] [Google Scholar]
- Das G. D. Gliogenesis and ependymogenesis during embryonic development of the rat. An autoradiographic study. J Neurol Sci. 1979 Oct;43(2):193–204. doi: 10.1016/0022-510x(79)90115-1. [DOI] [PubMed] [Google Scholar]
- Egar M., Singer M. The role of ependyma in spinal cord regeneration in the urodele, Triturus. Exp Neurol. 1972 Nov;37(2):422–430. doi: 10.1016/0014-4886(72)90085-4. [DOI] [PubMed] [Google Scholar]
- Garfia A., Mestres P., Rascher K. Trinitrophenol lesions of the ventricular wall: a SEM-TEM study. Scan Electron Microsc. 1980;(3):449–456. [PubMed] [Google Scholar]
- Gibson S. J., Polak J. M., Bloom S. R., Wall P. D. The distribution of nine peptides in rat spinal cord with special emphasis on the substantia gelatinosa and on the area around the central canal (lamina X). J Comp Neurol. 1981 Sep 1;201(1):65–79. doi: 10.1002/cne.902010106. [DOI] [PubMed] [Google Scholar]
- Gilmore S. A., Leiting J. E. Changes in the central canal area of immature rats following spinal cord injury. Brain Res. 1980 Nov 10;201(1):185–189. doi: 10.1016/0006-8993(80)90783-0. [DOI] [PubMed] [Google Scholar]
- Gilmore S. A. Neuroglial population in the spinal white matter of neonatal and early postnatal rats: an autoradiographic study of numbers of neuroglia and changes in their proliferative activity. Anat Rec. 1971 Oct;171(2):283–291. doi: 10.1002/ar.1091710208. [DOI] [PubMed] [Google Scholar]
- Gilmore S. A., Sims T. J., Leiting J. E. Central canal area in the early postnatal rat: normal development and radiation-induced changes. Brain Res. 1984 Jun;316(2):149–157. doi: 10.1016/0165-3806(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Håkansson C. H., von Mecklenburg C. The effects of ionizing irradiation on the ciliated cells of the central nervous system (CNS) in man--a scanning electron microscopy study. Scan Electron Microsc. 1981;4:93–98. [PubMed] [Google Scholar]
- Jaeger C. B., Teitelman G., Joh T. H., Albert V. R., Park D. H., Reis D. J. Some neurons of the rat central nervous system contain aromatic-L-amino-acid decarboxylase but not monoamines. Science. 1983 Mar 11;219(4589):1233–1235. doi: 10.1126/science.6131537. [DOI] [PubMed] [Google Scholar]
- Kerns J. M., Hinsman E. J. Neuroglial response to sciatic neurectomy. I. Light microscopy and autoradiography. J Comp Neurol. 1973 Oct 1;151(3):237–254. doi: 10.1002/cne.901510303. [DOI] [PubMed] [Google Scholar]
- Kohno K. Electron microscopic studies on Reissner's fiber and the ependymal cells in the spinal cord of the rat. Z Zellforsch Mikrosk Anat. 1969;94(4):565–573. doi: 10.1007/BF00936062. [DOI] [PubMed] [Google Scholar]
- Kriebel R. M. Ependymal cells of the filum terminale in fish (Poecilia sphenops) adapted to freshwater and saltwater: electron microscopic study. Anat Rec. 1981 Sep;201(1):189–195. doi: 10.1002/ar.1092010120. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., St Onge M. F., Faciane C. L. An electron microscopic analysis of abnormal ependymal cell proliferation and envelopment of sprouting axons following spinal cord transection in the rat. Acta Neuropathol. 1979 Jan 12;45(1):27–36. doi: 10.1007/BF00691801. [DOI] [PubMed] [Google Scholar]
- Morgan C., Nadelhaft I., de Groat W. C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981 Sep 20;201(3):415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- Nahin R. L., Madsen A. M., Giesler G. J., Jr Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J Comp Neurol. 1983 Nov 1;220(3):321–335. doi: 10.1002/cne.902200306. [DOI] [PubMed] [Google Scholar]
- Neuhuber W. The central projections of visceral primary afferent neurons of the inferior mesenteric plexus and hypogastric nerve and the location of the related sensory and preganglionic sympathetic cell bodies in the rat. Anat Embryol (Berl) 1982;164(3):413–425. doi: 10.1007/BF00315762. [DOI] [PubMed] [Google Scholar]
- Nordlander R. H., Singer M. The role of ependyma in regeneration of the spinal cord in the urodele amphibian tail. J Comp Neurol. 1978 Jul 15;180(2):349–374. doi: 10.1002/cne.901800211. [DOI] [PubMed] [Google Scholar]
- Pilkington G. J., Lantos P. L. The fine structure of stratified ependyma in the ventricular wall of N-ethyl-N-nitrosourea-treated rats. Acta Neuropathol. 1979 May 15;46(3):173–176. doi: 10.1007/BF00690840. [DOI] [PubMed] [Google Scholar]
- Rafols J. A., Goshgarian H. G. Spinal tanycytes in the adult rat: a correlative Golgi gold-toning study. Anat Rec. 1985 Jan;211(1):75–86. doi: 10.1002/ar.1092110112. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Subcommissural organ and adjacent ependyma: autoradiographic study of their origin in the mouse brain. Am J Anat. 1968 Mar;122(2):317–335. doi: 10.1002/aja.1001220210. [DOI] [PubMed] [Google Scholar]
- Rascher K., Booz K. H., Nacimiento A. C., Donauer E. The ependyma of the cat central canal, with particular reference to its mitochondria-containing bulbs. Scan Electron Microsc. 1985;(Pt 1):231–238. [PubMed] [Google Scholar]
- SIMPSON S. B., Jr ANALYSIS OF TAIL REGENERATION IN THE LIZARD LYGOSOMA LATERALE. I. INITIATION OF REGENERATION AND CARTILAGE DIFFERENTIATION: THE ROLE OF EPENDYMA. J Morphol. 1964 May;114:425–435. doi: 10.1002/jmor.1051140305. [DOI] [PubMed] [Google Scholar]
- Schueren A. M., DeSantis M. Cellular heterogeneity in the ependymal layer of the chicken's lumbosacral spinal cord. Exp Neurol. 1985 Feb;87(2):387–391. doi: 10.1016/0014-4886(85)90230-4. [DOI] [PubMed] [Google Scholar]
- Seitz R., Löhler J., Schwendemann G. Ependyma and meninges of the spinal cord of the mouse. A light-and electron-microscopic study. Cell Tissue Res. 1981;220(1):61–72. doi: 10.1007/BF00209966. [DOI] [PubMed] [Google Scholar]
- Simpson S. B., Jr Morphology of the regenerated spinal cord in the lizard, Anolis carolinensis. J Comp Neurol. 1968 Oct;134(2):193–210. doi: 10.1002/cne.901340207. [DOI] [PubMed] [Google Scholar]
- Singer M., Nordlander R. H., Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979 May 1;185(1):1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Stoltenburg-Didinger G., Bienentreu R. Ependymal variations in the caudal spinal cord. Acta Neuropathol Suppl. 1981;7:386–388. doi: 10.1007/978-3-642-81553-9_110. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. An electron microscopic study of the development of the ependyma of the central canal of the mouse spinal cord. J Anat. 1981 Jan;132(Pt 1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Torvik A., Murthy V. S. The spinal cord central canal in kaolin-induced hydrocephalus. J Neurosurg. 1977 Sep;47(3):397–402. doi: 10.3171/jns.1977.47.3.0397. [DOI] [PubMed] [Google Scholar]
- Vaquero J., Ramiro M. J., Oya S., Cabezudo J. M. Ependymal reaction after experimental spinal cord injury. Acta Neurochir (Wien) 1981;55(3-4):295–302. doi: 10.1007/BF01808445. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I., Manzano e Silva M. J., van den Pol A. N. Cerebrospinal fluid-contacting neurons of the central canal and terminal ventricle in various vertebrates. Cell Tissue Res. 1983;231(3):615–621. doi: 10.1007/BF00218119. [DOI] [PubMed] [Google Scholar]
- Zamora A. J. The ependymal and glial configuration in the spinal cord of urodeles. Anat Embryol (Berl) 1978 Jul 17;154(1):67–82. doi: 10.1007/BF00317955. [DOI] [PubMed] [Google Scholar]
- Zamora A. J., Thiesson D. Tight junctions in the ependyma of the spinal cord of the urodele Pleurodeles waltlii. Anat Embryol (Berl) 1980;160(3):263–274. doi: 10.1007/BF00305107. [DOI] [PubMed] [Google Scholar]