Abstract
Leishmania promastigotes express an abundant cell surface glycoconjugate, lipophosphoglycan (LPG). LPG contains a polymer of the disaccharide-phosphate repeat unit Galβ1,4Manα1-PO4, shared by other developmentally regulated molecules implicated in parasite virulence. Functional complementation of a Leishmania donovani LPG-defective mutant (OB1) accumulating a truncated LPG containing only the Manα1-PO4 residue of the first repeat unit identified LPG3, the Leishmania homolog of the mammalian endoplasmic reticulum (ER) chaperone GRP94. LPG3 resembles GRP94, as it localizes to the parasite ER, and lpg3– mutants show defects including down-regulation of surface GPI-anchored proteins and mild effects on other glycoconjugates. LPG3 binds cellular proteins and its Leishmania infantum GRP94 ortholog is highly immunogenic, suggesting a potential role in directing the immune response. However, null lpg3– mutants grow normally, are completely defective in the synthesis of phosphoglycans, and the LPG3 mRNA is regulated developmentally but not by stress or heat. Thus the role of LPG3/GRP94 in Leishmania metabolism differs significantly from other eukaryotes. Like the other glycoconjugate synthetic pathways in this parasite, its activity is focused on molecules implicated in virulence rather than viability.
Keywords: glycoconjugate biosynthesis/GPI-anchored molecules/lipophosphoglycan/parasite virulence/trypanosomatid protozoan
Introduction
During its life cycle, the protozoan parasite Leishmania alternates between two distinct developmental stages and hosts. Promastigotes replicate in the midgut of sand flies as the procyclic stage, and upon entry into stationary phase concomitant with the digestion of the blood meal by the fly, differentiate into the infective metacyclic form that is transmitted by the next fly bite. In the mammal, metacyclic promastigotes avoid destruction by immune defenses and ultimately are ingested by macrophages, where they differentiate and proliferate as amastigotes within an acidified vacuole. Amongst the mechanisms employed for survival within the hosts, Leishmania relies on the expression of specialized, developmentally regulated glycoconjugates (Ilg et al., 1999; Ilgoutz and McConville, 2001; Turco et al., 2001). In promastigotes, the most abundant cell surface glycoconjugate is lipophosphoglycan (LPG), a complex glycolipid consisting of a phosphoglycan backbone structure of Gal(β1,4)Manα1-PO4 repeat units (or PG repeats), anchored to the membrane via a GPI anchor containing a unique, conserved glycan core (Figure 1). LPG has been implicated in many important steps in the Leishmania life cycle (Turco and Descoteaux, 1992; McConville and Ferguson, 1993; Handman, 1999), and a Leishmania major mutant lacking LPG alone in an otherwise virulent background is attenuated in sand fly, mouse and macrophage infections (Sacks et al., 2000; Späth et al., 2000).
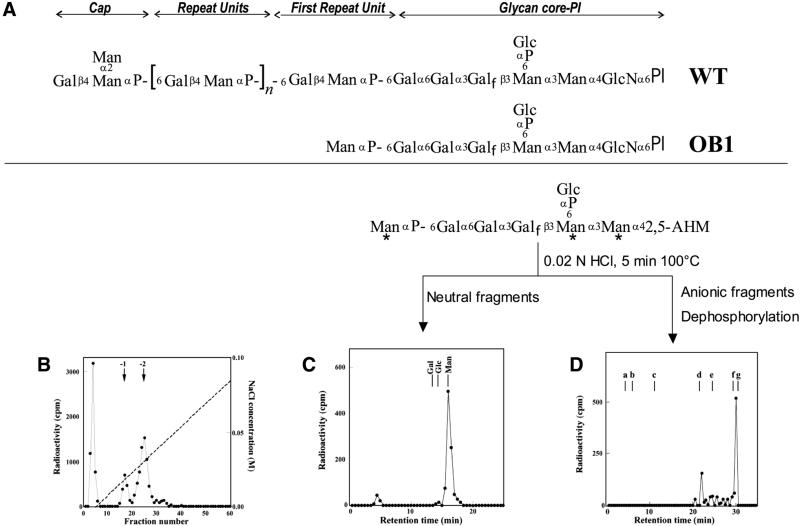
Fig. 1. Analysis of the truncated LPG accumulated in the L.donovani mutant OB1. (A) Structure of the LPG from L.donovani and OB1. (B) The [3H]mannose-labeled truncated LPG from OB1 was subjected to nitrous acid deamination and then applied to DEAE cellulose as described in Materials and methods. (C) The (–2) charged, [3H]mannose-labeled glycan from (B) (fractions 21–28) was hydrolyzed with mild acid into neutral and (–4) charged fragments, as depicted in the reaction scheme with the radioactive mannose residues indicated with asterisks, which were separated by anion exchange chromatography. The graph shows Dionex HPLC chromatography of the neutral fragment as described previously (Mahoney et al., 1999). The elution positions of standard monosaccharides are shown. (D) The (–4) charged fragment described in (C) was further treated with alkaline phosphatase and then analyzed by Dionex HPLC as described previously (Mahoney et al., 1999). Standards: a, Glc; b, Gal-Man; c, maltose; d, maltotriose; e, maltopentaose; f, maltohexaose; g, maltoheptaose.
In addition to LPG, Leishmania parasites abundantly synthesize a variety of complex glycoconjugates. These include a heterogeneous class of small glycosylinositolphospholipids (GIPLs), GPI anchored proteins such as gp63, gp46 and proteophosphoglycans (PPGs), and a variety of secreted proteins including acid phosphatase (sAP) and other forms of PPG (Handman, 1999; Ilg et al., 1999; McConville et al., 2002). Some of these are developmentally regulated, while others are constitutive, and like LPG, many have been proposed to be important to the execution of various steps of the parasite infectious cycle. One challenge in studying the biosynthetic pathways of these glycoconjugates and their role in virulence has been the finding that they share common structural domains. For example, the PG repeats of LPG are also added to secreted and membrane proteins including sAP and PPGs, and the LPG and GPI anchors of proteins have features in common with the GIPLs (references cited above).
Thus, we and others have made use of Leishmania mutants selected for specific defects in glycoconjugate expression to dissect out structure–function relationships and to identify LPG biosynthetic genes by functional rescue approaches (Ilg, 2001; Turco et al., 2001). These have proven useful in delineating the LPG biosynthetic pathway and its relationship to that of other glycoconjugates, and for generating clean ‘knockout’ mutants in a virulent background with defined effects on glycoconjugate synthesis. For example, mutants in the LPG1 gene affect only LPG, whereas mutations in LPG2 affect all phosphoglycan-containing glycoconjugates (Descoteaux et al., 1995; Ilg, 2000; Späth et al., 2000; Ilg et al., 2001; Turco et al., 2001). Interestingly, this role(s) appears to differ substantially amongst Leishmania species, with LPG and PG repeats playing important roles in survival in both the sand fly and macrophage stages in L.major and other species, but having little effect on macrophage survival in Leishmania mexicana, whereas viable mutants in other glycoconjugate synthetic pathways have strong virulence defects (Ilg, 2000; Späth et al., 2000; Garami and Ilg, 2001a,b; Garami et al., 2001; Ilg et al., 2001; Turco et al., 2001). These studies suggest that further study of the biosynthetic pathways leading to glycoconjugate assembly and expression could lead to the development of novel and more specific chemotherapeutic treatments against Leishmania.
Here we report on LPG3, a gene identified by functional complementation of the Leishmania donovani mutant OB1, which accumulates a truncated LPG that was characterized as Manα1-PO4-glycan core-PI. Remark ably, LPG3 encodes a homolog of the mammalian GRP94, a member of the HSP90 family of chaperones localized in the endoplasmic reticulum (ER) (Lee et al., 1984; Mazzarella and Green, 1987). In mammalian cells, GRP94 is a general chaperone associated with a variety of processes including antigen presentation, protein folding and secretion, and is required for viability (Li and Srivastava, 1993; Little et al., 1994; Nicchitta, 1998; Argon and Simen, 1999). Recently, a GRP94 homolog from Leishmania infantum was described, and was shown to be highly immunogenic similar to many HSP family members (Larreta et al., 2000), although the cellular role of this protein was not investigated. Our data show that while LPG3 shares some properties with GRP94, its functions within Leishmania appear to be highly divergent, as it is not required for normal growth and its role(s) and regulation differ considerably.
Results
Selection and characterization of the LPG-deficient mutant OB1
Wild-type (WT) L.donovani promastigotes were chemi cally mutagenized and selected for loss of agglutination by the β-galactose-binding lectin ricin agglutinin (King and Turco, 1988). From this population a clonal line was recovered that failed to react with ricin, named OB1. OB1 also was unreactive with the monoclonal antibody CA7AE, which recognizes the Gal(β1,4)Man(α1-PO4) PG repeating units from LPG (McNeely et al., 1990).
Glycoconjugate synthesis in OB1 was examined by metabolic labeling followed by extraction with organic solvents. As shown in Table I, low levels of [3H]mannose were incorporated into the LPG fraction (1.25% of WT), confirming that OB1 had lost the ability to synthesize LPG and that the loss of CA7AE reactivity was not due to conformational changes in LPG structure (Cappai et al., 1994). In contrast to the loss of LPG, [3H]mannose incorporation into GIPL fractions was only slightly reduced in OB1, and incorporation into glycoproteins was reduced by 50% (Table I).
Table I. [3H]mannose incorporation into glycoconjugates from WT and OB1 mutant L.donovani.
| Cell line | LPG fraction (%) | GIPLs fraction (%) | Glycoprotein fraction (%) |
|---|---|---|---|
| WT | 100 | 100 | 100 |
| OB1 | 1.25 ± 0.05 | 92 ± 34 | 51 ± 1.5 |
The average and range of two replicate experiments is shown, normalized to the parental WT L.donovani line (100%). In WT parasites the total c.p.m. incorporated into each fraction was: LPG, 146 000; GIPLs, 5000; and glycoproteins, 77 000. Parasites were labeled with [3H]mannose and the LPG, GIPL and glycoprotein fractions were prepared and incorporation quantitated as described in Materials and methods.
OB1 accumulates a truncated form of LPG
To determine the LPG biosynthetic defect in OB1, cells were metabolically labeled with [3H]mannose for 16 h. Glycolipids were extracted and delipidated with nitrous acid, and the deaminated products were separated by anion exchange chromatography. The 3H-labeled (–2) charged material (Figure 1B, fractions 21–28) was treated with mild acid hydrolysis and separated into two fragments, one of which co-migrated with standard mannose (Figure 1C). The other 3H fragment was found to be (–4) charged by anion exchange chromatography (data not shown). Following treatment with alkaline phosphatase, this fragment migrated with a retention time close to standard maltohexaose (Figure 1D, fraction 30) and identical to that of the dephosphorylated and deaminated glycan core of LPG (Gal-Gal-Galf-Man-Man-2,5-anhydromannose (Mahoney et al., 1999). A minor [3H]mannose-labeled peak (Figure 1D, fraction 24) co-migrating with standard maltotriose is probably the trisaccharide Man-Man-2,5 anhydromannose arising from partial hydrolysis at the labile Galf residue. These results indicated that the structure of the (–2) charged material (Figure 1B) corresponds to that arising from Man(α1-PO4)-glycan core-PI (Figure 1A). The neutral 3H-labeled material (Figure 1B, fractions 3–5) probably corresponds to the mannosylated GIPLs of L.donovani characterized previously (McConville and Blackwell, 1991). The (–1) charged 3H-labeled material (Figure 1B, fractions 15–20) was not studied further; it is most likely to be the same as the (–2) charged peak discussed above except for the lack of the Glc(α1-PO4) branch off the glycan core [Figure 1A; the LPG glycan core is known to be heterogeneous for this branch (McConville and Blackwell, 1991)]. Taken together, the data are consistent with the truncated form of LPG in OB1 as Man(α1-PO4)-glycan core-PI (Figure 1A).
OB1 cells lack GalT activity required for PG repeating unit synthesis
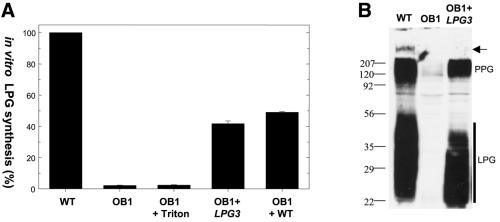
The results above suggest that OB1 has a defect in galactose attachment to the mannose residue within the PG repeating unit. We used a cell-free system to assay β1,4-GalT activity in OB1 membrane preparations, in which the transfer of radioactive UDP-Gal to endogenous LPG acceptors is measured (Carver and Turco, 1991; Huang and Turco, 1993). OB1 membranes completely lacked GalT activity; this was not restored by the addition of 0.1% Triton X-100 to disrupt membrane vesicles (Figure 2A), suggesting that the OB1 defect was not due to sequestration of the GalT activity within the microsome lumen. An equal mixture of WT and OB1 microsomal membranes (Figure 2A, OB1+WT) yielded 50% GalT activity, indicating that the OB1 defect was not due to the presence of an inhibitor or the deficiency of an activator. Collectively, these results suggested that OB1 lacked the GalT activity responsible for β-galactose addition to Man(α1-PO4)-glycan core-PI.
Fig. 2. Galactosyltransferase activity and LPG biosynthesis in the OB1 mutant. (A) Microsomal membranes were prepared from promastigotes of WT, OB1 and OB1 transfected with an LPG3 expression construct (OB1 + LPG3; cosOB1-AΔHindIII; see Figure 3C). Galactosyltransferase activity was assayed using endogenous LPG acceptors and UDP-[3H]galactose donor as described in Materials and methods, either directly, or in the presence of 0.1% Triton (OB1 + Triton), or of an equal mixture of WT and OB1 membranes (OB1 + WT). (B) Lysates corresponding to 106 log phase promastigotes were analyzed by western blotting with the anti-PG antibody CA7AE. The samples were WT, OB1 or OB1 transfected with an LPG3 expression construct (pXG-LPG3-GFP+). The arrow marks the interface between the stacking and separating gel, and the positions of PPG and LPG are marked.
Western blot analysis of total cell lysates from OB1 using a general anti-PG repeating unit antibody CA7AE showed a complete absence of LPG, as well as other PGs including those added to proteophosphoglycan (PPG) (Figure 2B; data not shown).
Functional rescue of OB1
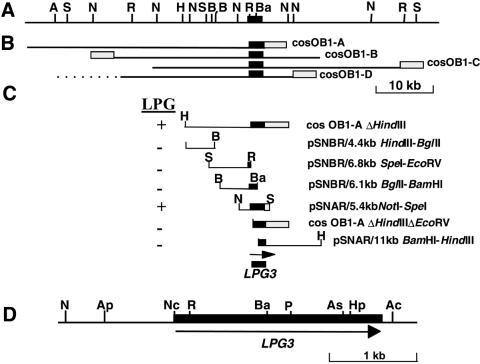
To identify the gene defective in OB1, mutant parasites were transfected with a WT L.donovani cosmid library, containing ∼40 kb random segments of genomic DNA inserted in the episomal shuttle cosmid vector cLHYG (Ryan et al., 1993a). Ten thousand independent transfectants were obtained and LPG+ transfectants were selected from these by two rounds of ricin panning. Four cosmids with maps overlapping by ∼17 kb were recovered (Figure 3A and B). Upon transfection into OB1, these cosmids were able to restore reactivity with ricin and CA7AE (data not shown). Microsomal membrane fractions from an OB1 transfectant bearing a cosOB1-A derivative showed 40–50% of WT GalT activity (Figure 2A, lane OB1+LPG3). Lastly, western blot analysis with CA7AE antibody showed restoration of LPG and PPG synthesis upon re-expression of LPG3 in OB1 (Figure 2B, lane OB1+LPG3).
Fig. 3. Functional mapping of the LPG3 locus. (A) Restriction map of the LPG3 genomic region. The LPG3 coding sequence is represented by the black box. (B) Position of four overlapping cosmids obtained by functional complementation of OB1. The shaded box represents the vector portion of cLHYG. (C) Localization of LPG3. CosOB1-A was subjected to deletional analysis to localize the region encoding the OB1-complementing gene (cosOB1-AΔHindIII and cosOB1-AΔHindIIIΔEcoRV). Various restriction fragments from cosOB1-AΔHindIII were subcloned in either Leishmania shuttle vectors pSNBR or pSNAR (Callahan and Beverley, 1991) and tested for their ability to restore LPG repeat unit expression in OB1 cells. An 11 kb BamHI–HindIII fragment from cosOB1-C subcloned into pSNAR was also tested. (D) Organization of the LPG3 locus. The LPG3 ORF is represented by a black box. Restriction enzymes: A, AflIII; Ac, AccI; Ap, ApaI; As, AscI; B, BglII; Ba, BamHI; H, HindIII; Hp, HpaI; N, NotI; Nc, NcoI; P, PvuI; R, EcoRV; S, SpeI.
Identification of the LPG3 gene
We tested a series of deletions of cosOB1-A, or of subcloned segments inserted into the Leishmania shuttle vector pSNBR (Callahan and Beverley, 1991). These data mapped the active gene to a segment spanning an EcoRV site adjacent to the cLHYG backbone, which was contained on a 5.4 kb NotI–SpeI fragment (Figure 3C, pSNAR 5.4 kb NotI–SpeI; this fragment additionally contained 1.9 kb of the cLHYG vector). Sequence analysis of the relevant region revealed an 813 amino acid reading frame, the last 43 amino acids of which arose from fusion to the vector cLHYG sequences. The remaining portion of this open reading frame (ORF) was obtained by sequencing the relevant region derived from cosOB1-B. Ultimately, we sequenced a 3.7 kb region that contained a 771 amino acid ORF, designated LPG3 (DDBJ/EMBL/GenBank accession No. AF369892; Figure 4). As shown below, this reading frame alone was able to restore LPG synthesis of OB1. In contrast, expression of mouse GRP94 in OB1 did not restore LPG synthesis (Materials and methods; data not shown).
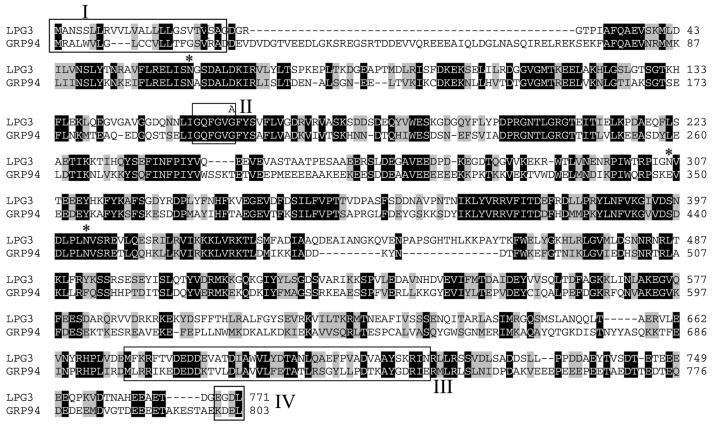
Fig. 4. Sequence of LPG3 and alignment with human GRP94. The sequences of LPG3 and human GRP94 proteins (Maki et al., 1990) were aligned using the ClustalW program. Black boxes indicate identity and shaded boxes indicate similarity. In one OB1 LPG3 allele, guanine 476 has been mutated into an adenine, resulting in the substitution of Gly159 for Asp159 (shown above LPG3 sequence). The GRP94 (Argon and Simen, 1999) and LPG3 signal sequences [box I; predicted by the SignalP algorithm; (Nielsen et al., 1997)] are underlined. The GXXGXG motif (box II) present within the putative nucleotide-binding region (amino acids 35–274) is conserved in all Hsp90 family proteins (Schulte et al., 1999). The dimerization domain of GRP94 (Argon and Simen, 1999) is shown in box III. The C-terminal KDEL tetrapeptide (box IV) is required for ER targeting of GRP94 (Munro and Pelham, 1987). Potential N-linked glycosylation sites in the mature LPG3 protein are marked by asterisks.
Database comparisons showed that the predicted LPG3 protein was closely related to the GRP94/HSP90 protein family, with 42% identity and 60% similarity to human GRP94 (Figure 4), and 97.7% identity to a GRP94 homolog recently described in L.infantum (Maki et al., 1990; Larreta et al., 2000). GRP94, a molecular chaperone belonging to the HSP90 family, is an abundant resident protein of the ER (Little et al., 1994; Argon and Simen, 1999). The predicted LPG3 protein exhibited many hallmarks of the GRP94 protein family, including an N-terminal signal peptide (Box I; residues 1–21), nucleotide binding domain (Box II; residues 154–159), several N-linked glycosylation sites in the mature protein (residues 63, 306 and 402) and the GRP94 dimerization domain located at positions 673–716 in LPG3 (Box III; Figure 4).
Cellular localization of LPG3
Notably, the predicted L.donovani LPG3 protein has a C-terminal EGDL sequence, which differs from the canonical KDEL ER retrieval signal of GRP94 and other ER resident proteins of other eukaryotes (Mazzarella and Green, 1987). The related L.infantum GRP94 gene contains EDDL at its C-terminus (Larreta et al., 2000). While the requirements for the ER retrieval signal have not been studied in Leishmania, data from the related species Trypanosoma brucei suggest that the requirements may be more relaxed than in higher eukaryotes, with sequences such as MDDL or KQDL showing ER retention activity (Bangs et al., 1996).
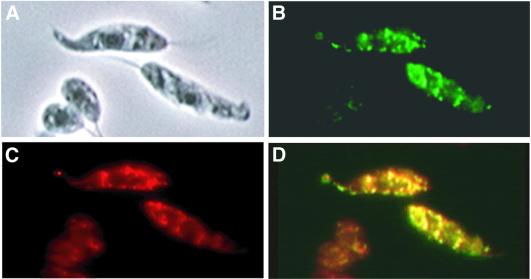
We created an LPG3–GFP fusion protein gene and ex pressed it in the OB1 mutant; these transfectants synthe sized LPG, as judged by restoration of ricin and CA7AE reactivity and western blot analysis (Figure 2B). Fluores cence microscopy showed that the LPG3–GFP fusion pro tein was localized to the Leishmania ER, as seen by the co-localization of the LPG3–GFP with BIP/GRP78, a known ER resident protein (Bangs et al., 1993; Jensen et al., 2001; Figure 5). Western blot and immunoprecipitation studies with anti-GFP antisera described below revealed that the LPG3–GFP fusion protein was intact. An ER localization of the native L.infantum GRP94 protein has also been suggested using anti-GRP94 antisera (J.Requena, personal communication), in agreement with the GFP-tagged LPG3/GRP94.
Fig. 5. LPG3–GFP is localized to the ER. WT L.donovani transfected with pXG-LPG3-GFP were grown in medium containing 50 µg/ml G418. (A) Phase contrast; (B) GFP fluorescence; (C) immunofluorescent staining with rabbit anti-T.brucei BIP antibody (1:1000) followed by Texas Red- labeled goat anti-rabbit IgG (1:2000). (D) Merge of (B) and (C).
In the active, ER-localized LPG3–GFP fusion protein, the terminal EGDL sequence was placed internal to the C-terminal GFP tag. Similarly, several other active LPG3 expressing constructs have truncated termini and/or heterologous C-terminal extensions (cosOB1-A is one example; Figure 3A and B). Since the KDEL ER retrieval signal must be C-terminal for its function (Munro and Pelham, 1987), these data suggest LPG3 may utilize other signals to achieve ER localization in addition to the EGDL. Recently, COP-I-independent (e.g. non-KDEL) ER retrieval mechanisms have been described in mammalian cells (Girod et al., 1999; White et al., 1999).
Identification of a mutant LPG3 allele in OB1
Southern blot analysis of digested DNAs or Leishmania chromosomes separated by pulsed-field gel electrophoresis showed that LPG3 was a single copy gene located on a 1.2 Mb chromosome, and that there was no difference between OB1 and WT L.donovani (Figure 6B; data not shown). We did not detect other LPG3-related sequences in these experiments, nor did we detect more than one candidate LPG3 ortholog in the L.major or trypanosome genome project data (in contrast, many HSP90 family members were found). Given the close evolutionary relationship of the two Leishmania species (classified within the same species complex), these data indicate that L.donovani LPG3 is orthologous to L.infantum GRP94.
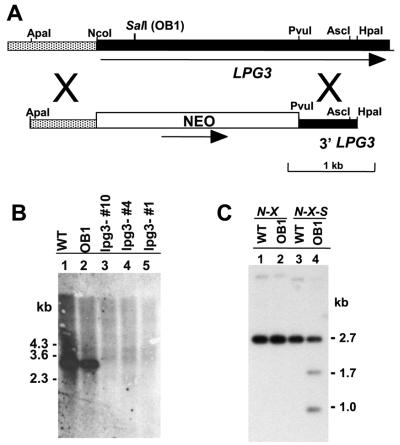
Fig. 6. Disruption of the LPG3 gene and analysis of OB1 LPG3 mutations. (A) Targeting construct for the disruption of LPG3. The open box represents the NEO resistance cassette, dark shading indicates the LPG3 ORF, and the hatched region represents 5′ flanking sequence. (B) Southern blot analysis of genomic DNA digested with NotI + AscI. The probe consisted of the 0.85 kb EcoRV–BamHI fragment from the LPG3 ORF (see Figure 3D). Lane 1, WT; lane 2, OB1; lanes 3–5, LPG-negative lpg3– null mutants. (C) Genomic DNA from WT (lanes 1 and 3) or OB1 (lanes 2 and 4) were digested with either NotI + XmaI (lanes 1 and 2) or NotI + XmaI + SalI (lanes 3 and 4). The restriction fragments were electrophoresed in a 0.85% agarose gel and transferred onto a Gene Screen Plus membrane. The membrane was probed with the 32P-labeled 1.0 kb NotI–BamHI fragment from the LPG3 locus (see Figure 3) spanning the mutated site (nucleotide 476) of the LPG3OB1 allele.
The similar Southern blot patterns between WT and OB1 suggested the possibility that rescue of the OB1 LPG defect was due to overexpression of LPG3 from the multicopy cosmid/plasmid vectors used, rather than by complementation. To test this, one of the OB1 LPG3 alleles was recovered from an OB1 genomic DNA cosmid library (cosLPG3OB1). When transfected into OB1, cosLPG3OB1 failed to restore LPG expression (data not shown), suggesting this was a loss-of-function allele. Sequence analysis of the LPG3OB1 gene revealed a single point mutation at nucleotide 476 (G→A), resulting in the substitution of Gly159 for Asp159 (Figure 4). Gly159 is located within the GRP94 nucleotide-binding domain (Argon and Simen, 1999), suggesting that this site is essential for LPG3 function(s) required for GalT activity.
Leishmania mutants frequently arise through a loss of heterozygosity mechanism (Gueiros-Filho and Beverley, 1996). Since the Gly159→Asp mutation also generated a new SalI restriction site, we used Southern blot analysis to show that OB1 cells were heterozygous at this site (Figure 6C). These data argue that either this LPG3OB1 allele was a dominant-negative mutant, or that the other allele contained a different inactivating mutation. Trans fection of cosLPG3OB1 in WT cells did not abolish LPG synthesis (data not shown), implying that LPG3OB1 was unlikely to be a dominant-negative mutant. While the sequence of the other allele in OB1 was not determined, in light of these data as well as the null mutant analysis below, we concluded that OB1 contains two independent mutations, each of which inactivated LPG3 function.
lpg3– null mutants are defective in LPG biosynthesis
To confirm that LPG3 was responsible for the LPG biosynthetic defect in OB1, we created an lpg3– null mutant by homologous gene replacement. A targeting construct in which the first 1.3 kb of the LPG3 ORF was replaced by a NEO cassette (Figure 6A) was introduced into WT parasites, and heterozygous replacements (LPG3/NEO) were obtained and confirmed by Southern blot analysis (data not shown). Next, homozygous knockouts were obtained by a loss of heterozygosity protocol as described in Materials and methods. Three clonal lines were obtained, all of which failed to react with CA7AE, and Southern blot analysis confirmed the loss of LPG3 (Figure 6B). Transfection of cosOB1-C into these null lpg3– mutants restored LPG expression, as revealed by reactivity with CA7AE.
Thus, loss of LPG3 is responsible for the LPG biosynthetic step defective in OB1 cells. Furthermore, in contrast to GRP94 in cultured mammalian cells or Dictyostelium (Little et al., 1994; Little and Lee, 1995; Morita et al., 2000), LPG3 was not required for growth in vitro in Leishmania, as the lpg3– knockouts grew at rates identical to WT.
LPG3 mRNA is regulated in the infectious cycle but not by stress
Northern blot analysis revealed that the LPG3 mRNA is expressed throughout the parasite life cycle as a 4 kb mRNA (Figure 7A). The LPG3 mRNA was down-regulated in WT stationary phase promastigotes (Figure 7A, lane 1 versus 2), and expressed at higher levels in amastigotes (lane 3). Interestingly, OB1 promastigotes contained increased levels of LPG3 mRNA in both log phase and stationary phase of growth (Figure 7A, lanes 4 and 5, respectively). This may represent an autoregulatory mechanism in response to loss of active LPG3 protein expression.
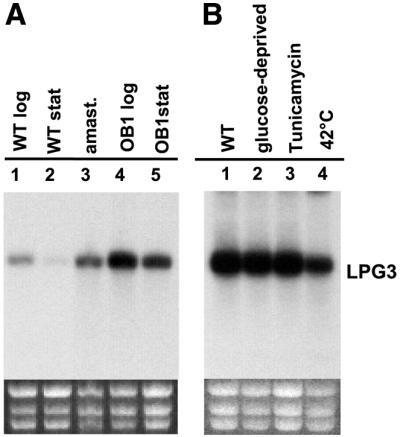
Fig. 7. Expression of LPG3 mRNA. Northern blot analysis was performed using (A) 5 µg or (B) 15 µg total RNA, and a 32P-labeled LPG3 coding region probe (0.85 kb EcoRV–BamHI or 1.05 kb NcoI–BamHI fragments, respectively; see Figure 3). The integrity and loading of the RNA is shown by the ribosomal RNA stained with ethidium bromide (bottom). (A) Developmental regulation of LPG3 mRNA. Lane 1, WT promastigotes in logarithmic phase; lane 2, WT promastigotes in stationary phase; lane 3, splenic amastigotes isolated from infected hamsters; lane 4, OB1 promastigotes in logarithmic phase; lane 5, OB1 promastigotes in stationary phase. (B) Effect of stresses on LPG3 mRNA. Total RNA was prepared from logarithmic phase WT promastigotes grown for 24 h at 26°C in either complete medium (lane 1), glucose-free medium (lane 2), complete medium in the presence of 5 µg/ml tunicamycin (lane 3) or for 4 h at 42°C in complete medium (lane 4).
A hallmark of mammalian GRP94 is the induction of its mRNA in the absence of glucose and by stress conditions that interfere with protein modification/folding or damage the ER (Little et al., 1994; Argon and Simen, 1999). However, exposure of WT log phase promastigotes to stress conditions known to induce GRP94 mRNA in mammalian cells (glucose deprivation, tunicamycin) had no effect on LPG3 mRNA levels (Figure 7B, lane 1 versus lanes 2 and 3). Moreover, the down-regulation of LPG3 mRNA observed in heat-shocked log-phase promastigotes (Figure 7B, lane 4) suggested that LPG3 may not be Leishmania HSP, as it is known that both HSP70 and HSP83 mRNA levels increase during heat-shock in these parasites [Shapira et al., 1988; this was confirmed by rehybridization of the northern blot shown in Figure 7B (data not shown)]. However, the relationship between mRNA and protein level for several Leishmania HSPs is complex (Shapira et al., 2001), and further studies at the protein level will be required to confirm this finding.
Role of LPG3 in the synthesis of secreted and surface Leishmania proteins
In mammalian cells, GRP94 is involved in essential processes of protein sorting and secretion (Little et al., 1994; Little and Lee, 1995). As shown earlier, while the LPG3 mutant OB1 mutant showed severe loss of PG synthesis involving both LPG and PPG, the effects on the synthesis of total mannosylated GIPLs or glycoproteins were modest (Table I; Figure 2B). We extended these studies to two important classes of Leishmania proteins: sAP, which shares the PG repeating units borne by LPG and PPGs, and the surface GPI-anchored proteins gp63 and gp46.
Leishmania sAP is known to be N-glycosylated in addition to containing PG repeats, with the former but not the latter modifications being essential for activity (Lovelace and Gottlieb, 1987; Bates et al., 1990; Descoteaux et al., 1995). Culture supernatants of OB1 showed a modest 2-fold reduction in sAP activity; however, this was not reversed following re-expression of LPG3 and restoration of LPG biosynthesis (Figure 8A). This suggested that the decrease was not related to LPG3 expression and probably arose from clonal variability associated with long-term in vitro culture and mutagenesis. In contrast, LPG3 was required for PG addition to sAP, as an antigen capture assay with the anti-PG antibody CA7AE showed that the sAP of the OB1 LPG3 mutant failed to bind at all (Figure 8B). Loss of PG repeating units was also evident in native gel electrophoresis, with OB1 sAP showing an increased mobility relative to WT, approaching that of the sAP of the C3PO mutant, which lacks all PGs (Figure 8C; Descoteaux et al., 1995). The intermediate mobility of OB1 sAP suggests that it is partially modified, possibly with the incomplete PG repeat units (Manα1-PO4 instead of Galβ1,4-Manα1-PO4) as seen with LPG (Figure 1A). In both antigen capture and mobility assays, the properties of sAP were restored to that of WT upon re-expression of LPG3 (Figure 8C, lane 3). Thus, while loss of LPG3 has little effect on sAP secretion or activity, it is required for PG addition.
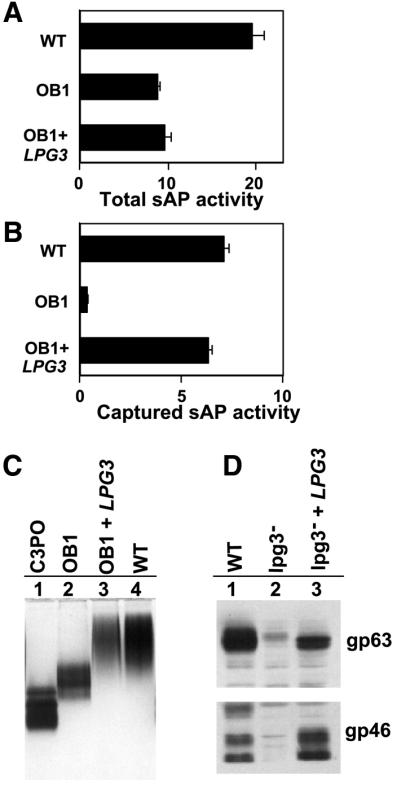
Fig. 8. Analysis of glycoconjugate expression in lpg3– mutants. (A) Secretion of active acid phosphatase (sAP) is independent of LPG3. Culture supernatant (100 µl) from log phase WT, OB1 or OB1 transfected with an LPG3 expression construct (cosOB1-AΔHindIII; see Figure 3) was assayed for acid phosphatase activity (Descoteaux et al., 1994; Privé and Descoteaux, 2000). sAP activity is expressed as nmol/min/ml/107 cells. (B) LPG3 is required for PG addition to sAP. An antigen capture assay was performed with the CA7AE anti-PG repeating unit antibody (Tolson et al., 1989) using 100 µl of culture supernatants described in (A); sAP activity was assayed and quantitated as in (A). (C) sAP mobility in non-denaturing gel electrophoresis. Culture supernatants were concentrated (5.5-fold) from C3PO (lane 1), WT (lane 2), OB1 (lane 3) and OB1 transfected with an LPG3 expression construct (lane 4; cosOB1-AΔHindIII; see Figure 3) and separated by electrophoresis in 7% acrylamide gels in the absence of SDS. The gel was stained for acid phosphatase activity as described previously (Descoteaux et al., 1995). (D) Effect of LPG3 on gp63 and gp46 levels. Gp63 (A) and gp46 (B) levels were assessed by western blots using anti-gp63 or or anti-gp46 antisera as described in the Materials and methods, using protein extracts from WT promastigotes (lane 1), lpg3– promastigotes (lane 2) and lpg3– promastigotes transfected with an LPG3 expression construct (lane 3).
In addition to the GPI-anchored glycolipids LPG and GIPLs, the Leishmania surface contains abundant levels of glycosylated GPI-anchored proteins, including PPG, gp63 and gp46 (Olafson et al., 1990; Schneider et al., 1990; Rivas et al., 1991). Unlike PPG or sAP, neither gp63 nor gp46 contains PG repeating units. Western blot analysis with appropriate sera showed that in contrast to the results obtained with sAP above, both gp63 and gp46 levels were significantly reduced in the lpg3– null mutant, although the mobility of the residual protein was similar to that of WT (Figure 8D). Re-expression of LPG3 expression restored gp63 and gp46 levels to that of WT (Figure 8D).
LPG3 binds to itself and at least one other Leishmania protein, but not to LPG
The relationship of LPG3 to GRP94 and the pleiotropic effects of LPG3 mutations on PG-bearing molecules (LPG, sAP, PPGs) and GPI-anchored protein synthesis suggested that LPG3 might function as a molecular chaperone, with specificity for protein or possibly nascent PGs. Several experiments were performed to search for binding of a protein with the predicted size of LPG3 to LPG, using total labeled proteins from WT or OB1 cells, but we were unable to detect specific binding that differed between the two lines (data not shown). Thus we sought evidence for specific protein binding by immunoprecipitation of LPG3 protein complexes. We used the active C-terminal GFP-tagged LPG3 protein described previously, and a second version bearing the OB1 mutant allele G159A. These proteins were expressed using the pXG expression vector in WT and OB1 parasites; the WT LPG3–GFP protein was functional as it was able to restore LPG expression in OB1 mutants, while the LPG3OB1–GFP protein was inactive (data not shown).
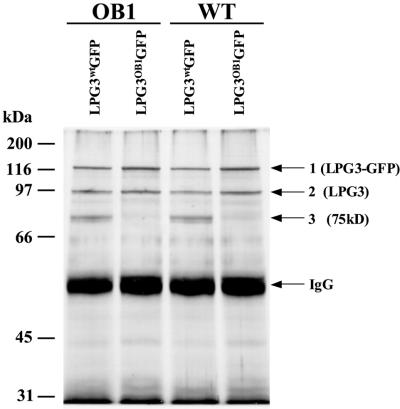
Lysates were prepared from the four transfectants, immunoprecipitated with an anti-GFP antibody, and proteins were separated by SDS–PAGE. In transfectants bearing LPG3wt–GFP, three major proteins with estimated sizes of 125, 92 and 75 kDa were identified by silver staining (Figure 9; similar results were obtained with [35S]Met/Cys in vivo-labeled cell lysates; data not shown). Notably, protein bands comparable in size to the GFP reporter or other degradation products were not observed in immunoprecipitation or western blot studies with the anti-GFP antisera, suggesting that the LPG3–GFP fusion protein was largely intact (data not shown). The 125 kDa band corresponded to the predicted 112 kDa LPG3–GFP fusion protein, as shown by western blot analysis with anti-GFP antibody (data not shown). The apparent size of the 92 kDa protein was similar to that of L.infantum GRP94 (Larreta et al., 2000) and that expected for mature LPG3 (calculated to be 85 kDa). Immunoprecipitation of native LPG3 by the LPG3–GFP fusion protein suggests that LPG3 exists in oligomeric complexes, like mammalian GRP94, which is known to exist as a dimer (Lewis et al., 1985; Qu et al., 1994). Both the LPG3–GFP and LPG3 proteins were immunoprecipitated from both OB1 and WT lysates, with either the LPG3WT–GFP and LPG3OB1–GFP fusion proteins. This is consistent with the location of the LPG3-OB1 mutation in the nucleotide-binding region, which is distinct from the GRP94 dimerization domain (Figure 4).
Fig. 9. Binding of Leishmania proteins to an LPG3–GFP fusion. Lysates from OB1 and WT Leishmania cells transfected with either pXG-LPG3wt-GFP or pXG-LPG3OB1-GFP were immunoprecipitated with anti-GFP antibodies (Clontech) and protein A–Sepharose as described in Materials and methods. Samples were subjected to SDS–PAGE (10% gel) under reducing conditions and silver-stained to visualize protein bands. Three distinct proteins are visible and labeled 1, 2 and 3. Band 1 corresponds to the LPG3–GFP fusion protein and band 2 has a size similar to the predicted LPG3 protein as discussed in the text. The identity of the third band (75 kDa) is unknown.
Significantly, a 75 kDa protein band was immunoprecipitated only by the LPG3WT–GFP protein, but not by the LPG3OB1–GFP protein, in both WT and OB1 transfectants. Thus, the LPG3OB1 nucleotide binding domain mutation appears to preclude the interaction between LPG3 and this protein. The identity of this protein remains to be determined; its mobility suggests that it is unlikely to be gp46, gp63 or other known or abundant Leishmania proteins, and potentially it is involved in one or more steps of glycoconjugate synthesis defective in OB1.
Discussion
In this report we have characterized the biochemical defect associated with LPG assembly in the L.donovani mutant OB1, and shown that it affects the addition of the galactosyl residue within the first PG repeat added to the nascent LPG chain (Figure 1). We used a functional complementation and gene knockout approach to identify LPG3 as the gene responsible for this defect. Remarkably, rather than encoding a PG-specific GalT as we had supposed, LPG3 encodes a member of the GRP94/HSP90 protein family, which are chaperones whose multiple functions include protein assembly and secretion, and antigen presentation (Melnick and Argon, 1995; Nicchitta, 1998; Argon and Simen, 1999). The homology to mammalian GRP94 spans the entire molecule and includes domains implicated in secretion, nucleotide binding and dimerization (Figure 4). Our data, as well as searches of the Leishmania genome databases, suggest that the L.infantum GRP94 gene, identified previously on the basis of its homology to mammalian GRP94, is orthologous to L.donovani LPG3.
Like other GRP94s, LPG3 is targeted to the parasite ER, although our data suggest that the mechanism by which this occurs may use mechanisms in addition to the canonical KDEL ER retrieval signal characterized previously for other ER resident proteins of trypanosomes and other eukaryotes (Munro and Pelham, 1987; Bangs et al., 1996). In addition, LPG3 shares many functional properties with GRP94, as lpg3– mutants show pleiotropic defects, including down-regulation of surface GPI-anchored glycoproteins and mild effects on N-glycosylation and glycolipid synthesis (Table I; Figures 2 and 8). While many chaperones show a broad ability to bind to many nascent proteins, mammalian GRP94s bind to a more limited set of proteins (Argon and Simen, 1999). Similarly, Leishmania LPG3 can form complexes with a small number of proteins, including itself and at least one unidentified 75 kDa protein (Figure 9). It is possible that this protein may be involved in one of the defects evident in the lpg3– mutants, such as GPI protein or PG galactosyltransferase activity, and we are investigating this question. Since PG biosynthesis occurs in the Leishmania Golgi apparatus (Bates et al., 1990; Descoteaux et al., 1995; Ha et al., 1996), the role of LPG3 in PG synthesis probably occurs by ensuring that at least one of the LPG biosynthetic enzymes is properly folded in the ER prior to transit. Although the GalT responsible for PG repeat synthesis has not been identified, searches of Leishmania databases of putative galactosyltransferase genes, as well as other likely glycosyltransferase genes, predict rather large proteins (90–160 kDa) compared with typical 40–50 kDa glycosyltransferases size found in other species (Field and Wainwright, 1995 and references therein). If relevant to the PG GalT, perhaps the larger size places more emphasis on LPG3/GRP94-dependent folding.
Recently, a GRP94 homolog from the related species L.infantum was identified and shown to be highly immunogenic, potentially implicating this protein in interactions involving the host immune system known to play important roles in parasite virulence (Locksley et al., 1999; Larreta et al., 2000, 2002). Indeed, reactivity to other molecular chaperones and HSPs has been implicated in the pathology of a number of microbial infections, including Leishmania (Requena et al., 2000; Srivastava, 2002). Interestingly, mammalian GRP94–peptide complexes have been shown to elicit cellular immune responses, an affect which can also be attained by exogenous administration of GRP94–peptide complexes (Nicchitta, 1998; Argon and Simen, 1999). This raises the possibility that complexes of Leishmania peptides with LPG3/GRP94 could interact with the host immune system and modulate immune responses, and this will be studied in the future.
Our data suggest that Leishmania LPG3/GRP94 shares many of the activities of GRP94s of other eukaryotes. However, despite these similarities, our most striking findings point to significant differences in the role of LPG3 in parasite metabolism. First, gene knockout studies show that LPG3 is not essential for parasite growth, whereas in mammalian cells or Dictyostelium, GRP94 is essential (Little and Lee, 1995; Morita et al., 2000). Secondly, the effects on protein secretion and glycosylation are considerably less profound. Thirdly, lpg3– mutants show severe defects in phosphoglycosylation, resulting in a complete lack of PG assembly on LPG and proteins. Fourthly, regulation of LPG3 mRNA expression differs greatly from that seen in mammalian cells, where the GRP94 mRNA typically is up-regulated in response to stress. In Leishmania, LPG3 mRNA expression appears to be regulated primarily in the context of development, with levels declining in stationary phase promastigotes where differentiation to the infectious metacyclic promastigote stage occurs, and increasing in amastigotes that reside within the macrophage phagolysosome and cause disease. Unlike mammalian GRP94 mRNA or HSP mRNAs in Leishmania and other organisms, the Leishmania LPG3 transcript is specifically not induced by canonical GRP94-inducing stresses such as heat, glucose deprivation or the glycosylation inhibitor tunicamycin (Figure 7B). Lastly, the LPG defect of the OB1 mutant could not be rescued by expression of mammalian GRP94.
Together, these data suggest that the role of LPG3 in Leishmania metabolism differs greatly from its homolog GRP94 in mammalian cells. Effectively, the critical role(s) of LPG3 is restricted to the synthesis of glycoconjugates implicated in parasite virulence, including LPG and other PG-bearing molecules, and GPI-anchored proteins. It is noteworthy that similar findings have emerged in studies of a number of Leishmania proteins associated typically with essential, ‘housekeeping’ functions of the secretory pathway in other eukaryotes. For example, Ilg and colleagues have shown that disruption of several genes of the mannose biosynthetic pathway required for protein glycosylation, such phosphomannomutase, phosphomannoisomerase, GDP-mannose pyrophosphorylase and dolichol-phosphate mannose synthase, have surprisingly little effect on parasite viability, yet have strong effects on the synthesis of glycoconjugates and virulence (Garami and Ilg, 2001a,b; Garami et al., 2001). Similarly, the Golgi GDP-mannose transporter is essential in Saccharomyces cerevisae (Poster and Dean, 1996), yet the Leishmania LPG2 gene encoding this transporter is dispensable, with adverse effects only upon PG biosynthesis and parasite virulence (Descoteaux et al., 1995; Ma et al., 1997; Ilg et al., 2001; Turco et al., 2001). Notably, the viability of parasite mutants in these genes provides opportunities for their study not possible in other eukaryotes where they are essential.
Prior to knowledge of the biochemical role of LPG2 in LPG and glycoconjugate synthesis, we had proposed that LPG2 is part of a specialized pathway leading to parasite virulence (Descoteaux et al., 1995). In light of recent knowledge of the role of LPG3 and many genes affecting glycoconjugate synthesis cited above, this hypothesis must be updated. Indeed, given the limited consequences in Leishmania of deletion of a broad spectrum of typically ‘essential’ eukaryotic genes affecting glycoconjugate structure and synthesis, it would appear that the specialization may involve the entire parasite secretory pathway, towards molecules important to parasite virulence, and away from molecules essential for viability as in most other eukaryotes. This does not imply that the basic function or role of the secretory pathway differs; instead, its focus seems to have shifted greatly, towards ‘cargo’ implicated in parasite survival within the host.
Materials and methods
Parasites and mutagenesis
Leishmania donovani 1S2D promastigotes were grown in M199 medium supplemented with 10% fetal calf serum (Descoteaux et al., 1994). Mutagenesis of promastigotes with 3 µg/ml of N-methyl-N-nitroso-N′-nitroguanidine and selection of LPG-defective mutants based on resistance to agglutination by the lectin ricin agglutinin were performed as described previously (King and Turco, 1988; Descoteaux et al., 1994).
Metabolic labeling and analysis of the truncated form of LPG from OB1
OB1 cells (1 × 109) were metabolically labeled with [2-3H]mannose (100 µCi) for 16 h in 2 ml of medium. [3H]GIPLs (some of which are the precursors to LPG) were extracted as described previously (McConville and Bacic, 1989). The GIPL extract was evaporated to dryness under N2, further purified by chromatography on phenyl–Sepharose, and delipidated by nitrous acid deamination (Huang and Turco, 1993). The deaminated glycan portion was collected by chromatography on phenyl–Sepharose, desalted on a column of Sephadex G25 equilibrated in water, resuspended in 1.0 mM Tris–HCl, pH 8.0, and applied to a column of DE52 cellulose (1× 2 ml) equilibrated in 1.0 mM Tris–HCl, pH 8.0. After the fifth fraction was collected, the column was eluted with a gradient of NaCl (0–0.1 M) in 1.0 mM Tris–HCl, pH 8.0. Fractions of 0.6 ml were collected and assayed for radioactivity by liquid scintillation counting. Following DEAE chromatography of the nitrous acid deaminated [3H]GIPLs, the [3H]mannose-labeled (–2) charged glycan was pooled and desalted. The [3H]glycan was subjected to mild acid hydrolysis in 0.02 N HCl for 5 min at 100°C to cleave the labile glycosylphosphate bonds, yielding neutral and (–4) charged fragments. The neutral fragment was subjected to Dionex HPLC. The (–4) charged fragment was further treated with alkaline phosphatase (0.3–0.5 U in 1 mM Tris) and then analyzed by Dionex HPLC as described previously (Mahoney et al., 1999).
Microsomal membrane preparation and GalT assays
Parasites were harvested at a density of 2–4 × 107 cells/ml and microsomal membranes were prepared (Carver and Turco, 1991). Incubation mixtures (400 µl) contained 0.4 mg of membrane protein, 50 mM HEPES (pH 7.4), 25 mM KCl, 5 mM MnCl2, 5 mM MgCl2, 0.1 mM Nα-p-tosyl-l-lysine chloromethyl ketone, 1 µg/ml leupeptin, 1 mM ATP, 0.5 mM dithiothreitol and 4 µCi UDP-[3H]Gal (specific activity, 15.3 Ci/mmol). In some reactions, Triton X-100 was added to 0.1% (v/v). The mixtures were incubated at 26°C for 1 h and the [3H-Gal]-LPG product was extracted, purified, and quantitated as described previously (Carver and Turco, 1991).
Western blotting
Log-phase parasites were washed twice in phosphate-buffered saline (PBS). For analysis of gp46 and gp63, parasites were suspended in ice-cold lysis buffer (20 mM Tris–HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl) containing protease inhibitors (Protease Inhibitor Cocktail, Roche Diagnostic). The extracts were incubated on ice for 15 min, vortexed and nuclei removed by centrifugation. Fifteen micrograms of protein/well were separated by SDS–PAGE on 8% polyacrylamide gels. For LPG, parasites were resuspended in SDS sample buffer (50 mM Tris–HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, 5% 2-mercaptoethanol) at 5 × 107 cells/ml. After incubation at 100°C for 5 min, lysates corresponding to 106 cells/lane were separated by SDS–PAGE on 10% polyacrylamide gels. Samples were electroblotted onto Hybond-ECL membranes (Amersham Pharmacia Biotech), and detected using an enhanced chemiluminescence method (Amersham Pharmacia Biotech). PG-containing glycoconjugates were detected with the anti-PG monoclonal antibody CA7AE which recognizes the Gal(β1–4)Manα1-PO4 repeating units (Tolson et al., 1989). gp63 was detected using the mouse monoclonal anti-gp63 (mAb 235; Button et al., 1991) and gp46 was detected using a rabbit antiserum to deglycosylated Leishmania amazonensis gp46 prepared and provided by D.McMahon-Pratt, Yale University Medical School (Champsi and McMahon-Pratt, 1988). Peroxidase-conjugated anti-rabbit and anti-mouse antisera were from Amersham Pharmacia Biotech.
Secreted acid phosphatase analysis
Supernatants from promastigote cultures (ranging from 8 to 10 × 106 cells/ml) were concentrated 5.5-fold using Centricon concentrators. Twenty-five microliters of each concentrated supernatant was electrophoresed in non-denaturing conditions in 7% acrylamide gels, which were subsequently stained for acid phosphatase activity (Katakura and Kobayashi, 1988). A PG repeat antigen capture assay was performed on promastigotes culture supernatants as described previously (Descoteaux et al., 1995) using the CA7AE antibody.
Fluorescence and immunofluorescence microscopy
WT L.donovani transfected with pXG-LPG3-GFP were grown in medium containing 50 µg/ml G418. Log phase cells were attached to poly-lysine coated cover slips and fixed in 3.5% formaldehyde (1 min at room temperature) followed by ethanol (15 min on ice). A rabbit anti-BIP polyclonal antibody (provided by E.Handman, Australia) was used to reveal the ER (1:1000, 20 min at room temperature), followed by goat anti-rabbit IgG-Texas Red labeled secondary antibody (1:2000).
Immunoprecipitation
Parasites (8 × 109 cells) were pelleted by centrifugation and washed once in ice-cold PBS. The cells were resuspended in 2.5 ml of prechilled lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM CaCl2, 5 µg/ml leupeptin, 2 µg/ml pepstatin A, 0.5% Triton X-100), and incubated for 30 min on ice. Lysates were clarified by centrifugation at 1000 g for 10 min and then 10 000 g for 10 min at 4°C. Immunoprecipitations were performed by adding 1–2 µl of rabbit polyclonal anti-GFP antibodies (Clontech) for 1 h at 4°C with shaking. Immune complexes were precipitated by the addition of 20 µl of protein A–Sepharose and 40 µl of Sepharose (Pharmacia) for 2 h followed by centrifugation for 20 s at 12 000 g. Immunoprecipitated samples were washed three to four times with lysis buffer, boiled in sample buffer (2% SDS, 60 mM Tris pH 6.8, 2.5% β-mercaptoethanol) and then centrifuged for 20 s at 12 000 g. The supernatants were subjected to SDS–PAGE (10% polyacrylamide containing 1% SDS) in the presence of β-mercaptoethanol, and the gels were silver-stained to visualize protein bands, or analyzed by western blotting as described above with anti-GFP antibodies (1:1000).
Parasite transfections and LPG+ selections
OB1 cells were transfected with WT L.donovani cosmid library DNA (Ryan et al., 1993a) and HYG-resistant tranfectants were selected as described previously (Ryan et al., 1993b; Descoteaux et al., 1994). LPG+ OB1 transfectants were selected by two rounds of LPG panning using 10 µg/ml of ricin agglutinin (Descoteaux et al., 1994). An OB1 genomic DNA cosmid library was constructed in cLHYG as described previously (Descoteaux et al., 1994). The library was probed with the 0.85 kb EcoRV–BamHI fragment from LPG3 (Figure 3) to identify cosmids containing the OB1 LPG3 gene.
LPG3 targeting construct and generation of lpg3– null mutants
The LPG3 deletion targeting construct (pLPG3-KO; strain D133) consisted of a 3.8 kb ApaI–HpaI fragment in which the first 1.3 kb of LPG3 coding sequence was replaced by a NEO cassette followed by the 3′ untranslated region of DHFR/TS (Figure 6A). This fragment was transfected into the WT line to generate G418-resistant clones with heterozygous LPG3 replacements (LPG3/Δlpg3::NEO). To obtain homozygous replacements, we used a loss-of-heterozygosity protocol (Descoteaux et al., 1995; Gueiros-Filho and Beverley, 1996). Heterozygote clones were grown in 20 µg/ml G418 and were submitted to five successive rounds of agglutination with the CA7AE monoclonal antibody followed by plating to obtain a clonal lpg3– line (Δlpg3::NEO/Δlpg3::NEO). LPG3 expression was restored in the lpg3– null mutant by transfection of several constructs including cosOB1-C (B1230), pXG-LPG3-GFP+ (below) or pXGSAT-LPG3 (B3366). This construct was made by ligation of the 2.3 kb LPG3 NcoI–NotI fragment from pXG-LPG3-GFP+ into SmaI–BamHI-digested pXGSAT (B2352), after all termini had been made blunt by the activity of the Klenow fragment of DNA polymerase I.
Expression of murine GRP94 in Leishmania
The mouse GRP94 ORF, contained on a 2.7 kb BamHI fragment, was excised from pGEM99.2 (Mazzarella and Green, 1987) and inserted into the BamHI site of pXG (Ha et al., 1996), to create pXG1-mGRP94 (B2283). This construct was transfected into OB1 and tested for LPG expression. Expression of mouse GRP94 in Leishmania cells was assessed by western blotting with a rabbit polyclonal antiserum raised against the 16 C-terminal amino acids of murine GRP94 (provided by M.Green, St Louis University, MO).
LPG3–GFP construct
An LPG3–GFP protein fusion expression vector (pXG-LPG3GFP; B-2396) was constructed using the pXG-’GFP vector (Ha et al., 1996; B-2295). The pXG-LPG3OB1GFP construct (D106) was obtained by replacing the 1.7 kb EcoRV–AscI fragment from pXG-LPG3GFP with the 1.7 kb EcoRV–AscI fragment from p8kbNsiI(OB1) (B-2193), which contains a blunted 8.0 kb NsiI fragment (containing the LPG3OB1 allele) from cosLPG3OB1 inserted into pUCπ digested with EcoRI and HindIII.
Acknowledgments
Acknowledgements
We thank D.Ma and M.Varin for experimental assistance; M.Green, D.McMahon-Pratt, J.Bangs, E.Handman, R.McMaster and L.Epstein for reagents; J.Requena for permission to mention results concerning the ER localization of L.infantum GRP94; and D.E.Dobson, E.Unanue and S.Virgin for discussions. This work was supported by National Institutes of Health grant AI31078 to S.M.B. and S.J.T., and partly by CIHR grant MT12933 to A.D. H.A.A. was supported by NIH postdoctoral fellowship AI09729. A.D. is a Scholar from the Fonds de la Recherche en Santé du Québec and Holder of a Canada Research Chair.
References
- Argon Y. and Simen,B.B. (1999) GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol., 10, 495–505. [DOI] [PubMed] [Google Scholar]
- Bangs J.D., Uyetake,L., Brickman,M.J., Balber,A.E. and Boothroyd,J.C. (1993) Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J. Cell Sci., 105, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Bangs J.D., Brouch,E.M., Ransom,D.M. and Roggy,J.L. (1996) A soluble secretory reporter system in Trypanosoma brucei. Studies on endoplasmic reticulum targeting. J. Biol. Chem., 271, 18387–18393. [DOI] [PubMed] [Google Scholar]
- Bates P.A., Hermes,I. and Dwyer,D.M. (1990) Golgi-mediated post-translational processing of secretory acid phosphatase by Leishmania donovani promastigotes. Mol. Biochem. Parasitol., 39, 247–255. [DOI] [PubMed] [Google Scholar]
- Button L.L., Reiner,N.E. and McMaster,W.R. (1991) Modification of GP63 genes from diverse species of Leishmania for expression of recombinant protein at high levels in Escherichia coli. Mol. Biochem. Parasitol., 44, 213–224. [DOI] [PubMed] [Google Scholar]
- Callahan H.L. and Beverley,S.M. (1991) Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J. Biol. Chem., 266, 18427–18430. [PubMed] [Google Scholar]
- Cappai R. et al. (1994) Ricin-resistant mutants of Leishmania major which express modified lipophosphoglycan remain infective for mice. Parasitology, 108, 397–405. [DOI] [PubMed] [Google Scholar]
- Carver M.A. and Turco,S.J. (1991) Cell-free biosynthesis of lipophosphoglycan from Leishmania donovani. Characterization of microsomal galactosyltransferase and mannosyltransferase activities. J. Biol. Chem., 266, 10974–10981. [PubMed] [Google Scholar]
- Champsi J. and McMahon-Pratt,D. (1988) Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect. Immun., 56, 3272–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A., Garraway,L.A., Ryan,K.A., Garrity,L.K., Turco,S.J. and Beverley,S.M. (1994) Identification of genes by functional complemenation in the protozoan parasite Leishmania. In Adolph,K.W. (ed.), Methods in Molecular Genetics. Vol. 3. Academic Press, San Diego, CA, pp. 22–48.
- Descoteaux A., Luo,Y., Turco,S.J. and Beverley,S.M. (1995) A specialized pathway affecting virulence glycoconjugates of Leishmania. Science, 269, 1869–1872. [DOI] [PubMed] [Google Scholar]
- Field M.C. and Wainwright,L.J. (1995) Molecular cloning of eukaryotic glycoprotein and glycolipid glycosyltransferases: a survey. Glycobiology, 5, 463–472. [DOI] [PubMed] [Google Scholar]
- Garami A. and Ilg,T. (2001a) Disruption of mannose activation in Leishmania mexicana: GDP-mannose pyrophosphorylase is required for virulence, but not for viability. EMBO J., 20, 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A. and Ilg,T. (2001b) The role of phosphomannose isomerase in Leishmania mexicana glycoconjugate synthesis and virulence. J. Biol. Chem., 276, 6566–6575. [DOI] [PubMed] [Google Scholar]
- Garami A., Mehlert,A. and Ilg,T. (2001) Glycosylation defects and virulence phenotypes of Leishmania mexicana phosphomannomutase and dolicholphosphate-mannose synthase gene deletion mutants. Mol. Cell. Biol., 21, 8168–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A., Storrie,B., Simpson,J.C., Johannes,L., Goud,B., Roberts,L.M., Lord,J.M., Nilsson,T. and Pepperkok,R. (1999) Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol., 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Gueiros-Filho F.J. and Beverley,S.M. (1996) Selection against the dihydrofolate reductase-thymidylate synthase (DHFR-TS) locus as a probe of genetic alterations in Leishmania major. Mol. Cell. Biol., 16, 5655–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha D.S., Schwarz,J.K., Turco,S.J. and Beverley,S.M. (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol., 77, 57–64. [DOI] [PubMed] [Google Scholar]
- Handman E. (1999) Cell biology of Leishmania. Adv. Parasitol., 44, 1–39. [DOI] [PubMed] [Google Scholar]
- Huang C. and Turco,S.J. (1993) Defective galactofuranose addition in lipophosphoglycan biosynthesis in a mutant of Leishmania donovani. J. Biol. Chem., 268, 24060–24066. [PubMed] [Google Scholar]
- Ilg T. (2000) Lipophosphoglycan is not required for infection of macrophages or mice by Leishmania mexicana. EMBO J., 19, 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg T. (2001) Lipophosphoglycan of the protozoan parasite Leishmania: stage- and species-specific importance for colonization of the sandfly vector, transmission and virulence to mammals. Med. Microbiol. Immunol. (Berl.), 190, 13–17. [DOI] [PubMed] [Google Scholar]
- Ilg T., Handman,E. and Stierhof,Y.D. (1999) Proteophosphoglycans from Leishmania promastigotes and amastigotes. Biochem. Soc. Trans., 27, 518–525. [DOI] [PubMed] [Google Scholar]
- Ilg T., Demar,M. and Harbecke,D. (2001) Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J. Biol. Chem., 276, 4988–4997. [DOI] [PubMed] [Google Scholar]
- Ilgoutz S.C. and McConville,M.J. (2001) Function and assembly of the Leishmania surface coat. Int. J. Parasitol., 31, 899–908. [DOI] [PubMed] [Google Scholar]
- Jensen A.T., Curtis,J., Montgomery,J., Handman,E. and Theander,T.G. (2001) Molecular and immunological characterisation of the glucose regulated protein 78 of Leishmania donovani (1). Biochim. Biophys. Acta, 1549, 73–87. [DOI] [PubMed] [Google Scholar]
- Katakura K. and Kobayashi,A. (1988) Acid phosphatase activity of virulent and avirulent clones of Leishmania donovani promastigotes. Infect. Immun., 56, 2856–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.L. and Turco,S.J. (1988) A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol. Biochem. Parasitol., 28, 285–293. [DOI] [PubMed] [Google Scholar]
- Larreta R., Soto,M., Alonso,C. and Requena,J.M. (2000) Leishmania infantum: gene cloning of the GRP94 homologue, its expression as recombinant protein and analysis of antigenicity. Exp. Parasitol., 96, 108–115. [DOI] [PubMed] [Google Scholar]
- Larreta R., Guzman,F., Patarroyo,M.E., Alonso,C. and Requena,J.M. (2002) Antigenic properties of the Leishmania infantum GRP94 and mapping of linear B-cell epitopes. Immunol. Lett., 80, 199–205. [DOI] [PubMed] [Google Scholar]
- Lee A.S., Bell,J. and Ting,J. (1984) Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem., 259, 4616–4621. [PubMed] [Google Scholar]
- Lewis M.J., Turco,S.J. and Green,M. (1985) Structure and assembly of the endoplasmic reticulum. Biosynthetic sorting of endoplasmic reticulum proteins. J. Biol. Chem., 260, 6926–6931. [PubMed] [Google Scholar]
- Li Z. and Srivastava,P.K. (1993) Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J., 12, 3143–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little E. and Lee,A.S. (1995) Generation of a mammalian cell line deficient in glucose-regulated protein stress induction through targeted ribozyme driven by a stress-inducible promoter. J. Biol. Chem., 270, 9526–9534. [PubMed] [Google Scholar]
- Little E., Ramakrishnan,M., Roy,B., Gazit,G. and Lee,A.S. (1994) The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation and applications. Crit. Rev. Eukaryot. Gene Expr., 4, 1–18. [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Pingel,S., Lacy,D., Wakil,A.E., Bix,M. and Fowell,D.J. (1999) Susceptibility to infectious diseases: Leishmania as a paradigm. J. Infect. Dis., 179 (Suppl. 2), S305–S308. [DOI] [PubMed] [Google Scholar]
- Lovelace J.K. and Gottlieb,M. (1987) Effect of tunicamycin on the extracellular acid phosphatase of Leishmania donovani promastigotes. Mol. Biochem. Parasitol., 22, 19–28. [DOI] [PubMed] [Google Scholar]
- Ma D.Q., Russell,D.G., Beverley,S.M. and Turco,S.J. (1997) Golgi GDP-mannose uptake requires Leishmania LPG2—A member of a eukaryotic family of putative nucleotide-sugar transporters. J. Biol. Chem., 272, 3799–3805. [PubMed] [Google Scholar]
- Mahoney A.B., Sacks,D.L., Saraiva,E., Modi,G. and Turco,S.J. (1999) Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani–sand fly interactions. Biochemistry, 38, 9813–9823. [DOI] [PubMed] [Google Scholar]
- Maki R.G., Old,L.J. and Srivastava,P.K. (1990) Human homologue of murine tumor rejection antigen gp96: 5′-regulatory and coding regions and relationship to stress-induced proteins. Proc. Natl Acad. Sci. USA, 87, 5658–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R.A. and Green,M. (1987) ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J. Biol. Chem., 262, 8875–8883. [PubMed] [Google Scholar]
- McConville M.J. and Bacic,A. (1989) A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization and antigenicity. J. Biol. Chem., 264, 757–766. [PubMed] [Google Scholar]
- McConville M.J. and Blackwell,J.M. (1991) Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J. Biol. Chem., 266, 15170–15179. [PubMed] [Google Scholar]
- McConville M.J. and Ferguson,M.A. (1993) The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J., 294, 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M.J., Mullin,K.A., Ilgoutz,S.C. and Teasdale,R.D. (2002) Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev., 66, 122–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely T.B., Tolson,D.L., Pearson,T.W. and Turco,S.J. (1990) Characterization of Leishmania donovani variant clones using anti-lipophosphoglycan monoclonal antibodies. Glycobiology, 1, 63–69. [DOI] [PubMed] [Google Scholar]
- Melnick J. and Argon,Y. (1995) Molecular chaperones and the biosynthesis of antigen receptors. Immunol. Today, 16, 243–250. [DOI] [PubMed] [Google Scholar]
- Morita T., Saitoh,K., Takagi,T. and Maeda,Y. (2000) Involvement of the glucose-regulated protein 94 (Dd-GRP94) in starvation response of Dictyostelium discoideum cells. Biochem. Biophys. Res. Commun., 274, 323–331. [DOI] [PubMed] [Google Scholar]
- Munro S. and Pelham,H.R. (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell, 48, 899–907. [DOI] [PubMed] [Google Scholar]
- Nicchitta C.V. (1998) Biochemical, cell biological and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/gp96. Curr. Opin. Immunol., 10, 103–109. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Engelbrecht,J., Brunak,S. and von Heijne,G. (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng., 10, 1–6. [DOI] [PubMed] [Google Scholar]
- Olafson R.W., Thomas,J.R., Ferguson,M.A.J., Dwek,R.A., Chaudhuri,M., Chang,K.-P. and Rademacher,T.W. (1990) Structures of the N-linked oligosaccharides of Gp63, the major surface glycoprotein, from Leishmania mexicana amazonensis. J. Biol. Chem., 265, 12240–12247. [PubMed] [Google Scholar]
- Poster J.B. and Dean,N. (1996) The yeast VRG4 gene is required for normal Golgi functions and defines a new family of related genes. J. Biol. Chem., 271, 3837–3845. [DOI] [PubMed] [Google Scholar]
- Privé C. and Descoteaux,A. (2000) Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38, c-Jun N-terminal kinase and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur. J. Immunol., 30, 2235–2244. [DOI] [PubMed] [Google Scholar]
- Qu D., Mazzarella,R.A. and Green,M. (1994) Analysis of the structure and synthesis of GRP94, an abundant stress protein of the endoplasmic reticulum. DNA Cell Biol., 13, 117–124. [DOI] [PubMed] [Google Scholar]
- Requena J.M., Alonso,C. and Soto,M. (2000) Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol. Today, 16, 246–250. [DOI] [PubMed] [Google Scholar]
- Rivas L., Kahl,L., Manson,K. and McMahon-Pratt,D. (1991) Biochemical characterization of the protective membrane glycoprotein GP46/M-2 of Leishmania amazonensis. Mol. Biochem. Parasitol., 47, 235–244. [DOI] [PubMed] [Google Scholar]
- Ryan K.A., Dasgupta,S. and Beverley,S.M. (1993a) Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene, 131, 145–150. [DOI] [PubMed] [Google Scholar]
- Ryan K.A., Garraway,L.A., Descoteaux,A., Turco,S.J. and Beverley,S.M. (1993b) Isolation of virulence genes directing surface glycosyl-phosphatidylinositol synthesis by functional com plementation of Leishmania. Proc. Natl Acad. Sci. USA, 90, 8609–8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D.L., Modi,G., Rowton,E., Späth,G., Epstein,L., Turco,S.J. and Beverley,S.M. (2000) The role of phosphoglycans in Leishmania–sand fly interactions. Proc. Natl Acad. Sci. USA, 97, 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Ferguson,M.A.J., McConville,M.J., Mehlert,A., Homans,S.W. and Bordier,C. (1990) Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J. Biol. Chem., 265, 16955–16964. [PubMed] [Google Scholar]
- Schulte T.W. et al. (1999) Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol., 13, 1435–1448. [DOI] [PubMed] [Google Scholar]
- Shapira M., McEwen,J.G. and Jaffe,C.L. (1988) Temperature effects on molecular processes which lead to stage differentiation in Leishmania. EMBO J., 7, 2895–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Zilka,A., Garlapati,S., Dahan,E., Dahan,I. and Yavesky,V. (2001) Post transcriptional control of gene expression in Leishmania. Med. Microbiol. Immunol. (Berl.), 190, 23–26. [DOI] [PubMed] [Google Scholar]
- Späth G.F., Epstein,L., Leader,B., Singer,S.M., Avila,H.A., Turco,S.J. and Beverley,S.M. (2000) Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite leishmania major. Proc. Natl Acad. Sci. USA, 97, 9258–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. (2002) Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol., 2, 185–194. [DOI] [PubMed] [Google Scholar]
- Tolson D.L., Turco,S.J., Beecroft,R.P. and Pearson,T.W. (1989) The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol. Biochem. Parasitol., 35, 109–118. [DOI] [PubMed] [Google Scholar]
- Turco S.J. and Descoteaux,A. (1992) The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol., 46, 65–94. [DOI] [PubMed] [Google Scholar]
- Turco S.J., Späth,G.F. and Beverley,S.M. (2001) Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol., 17, 223–226. [DOI] [PubMed] [Google Scholar]
- White J. et al. (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol., 147, 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]