Abstract
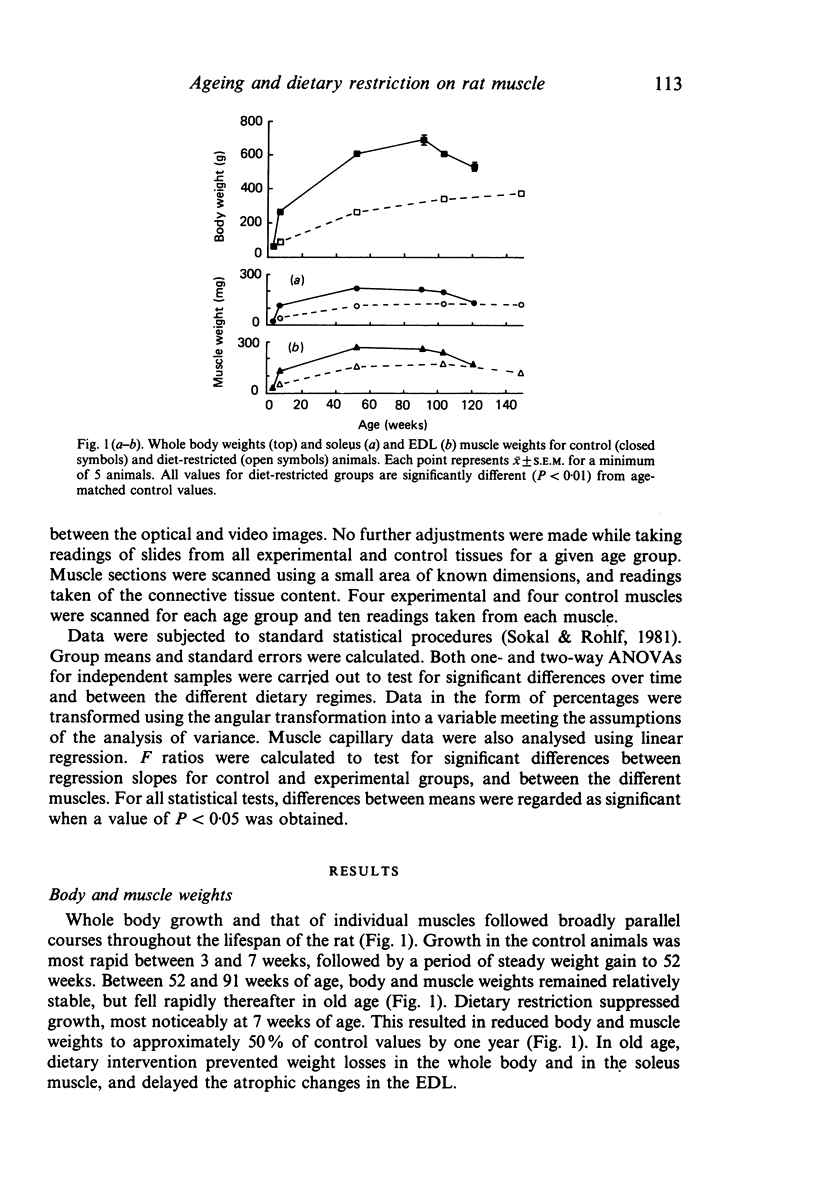
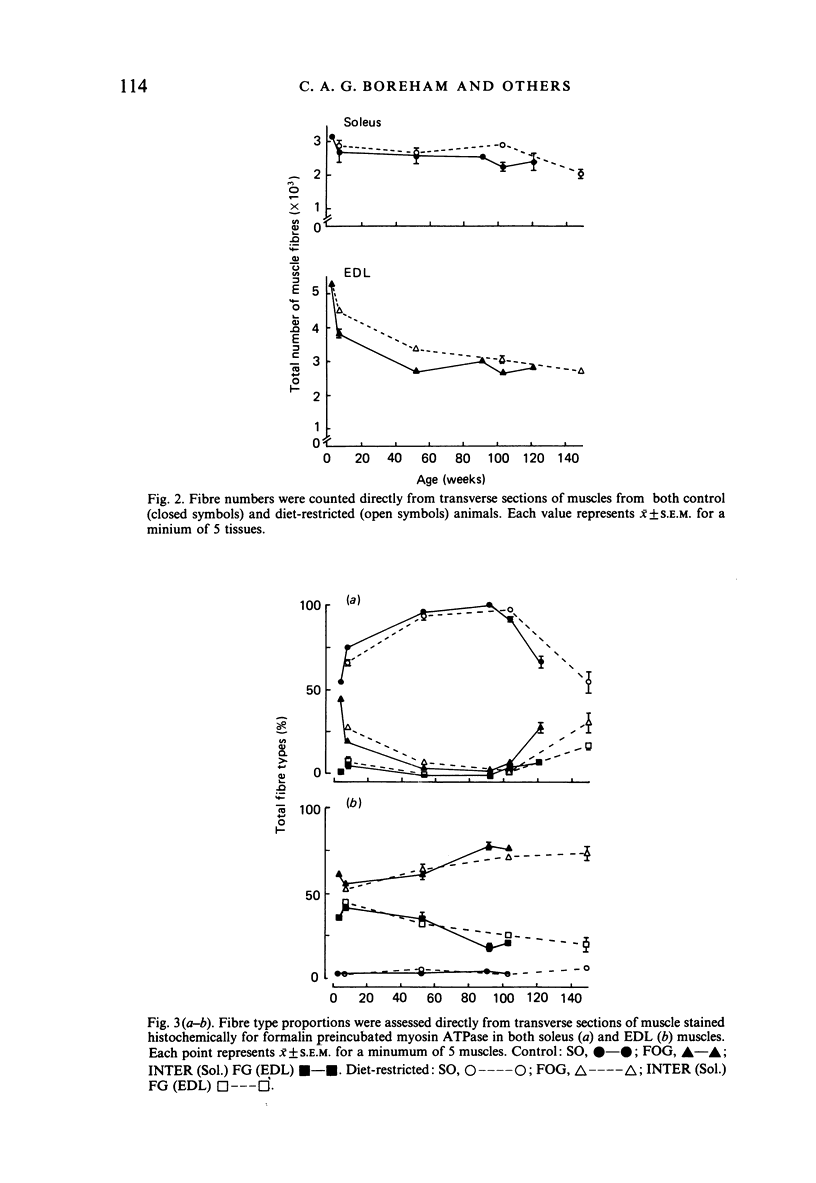
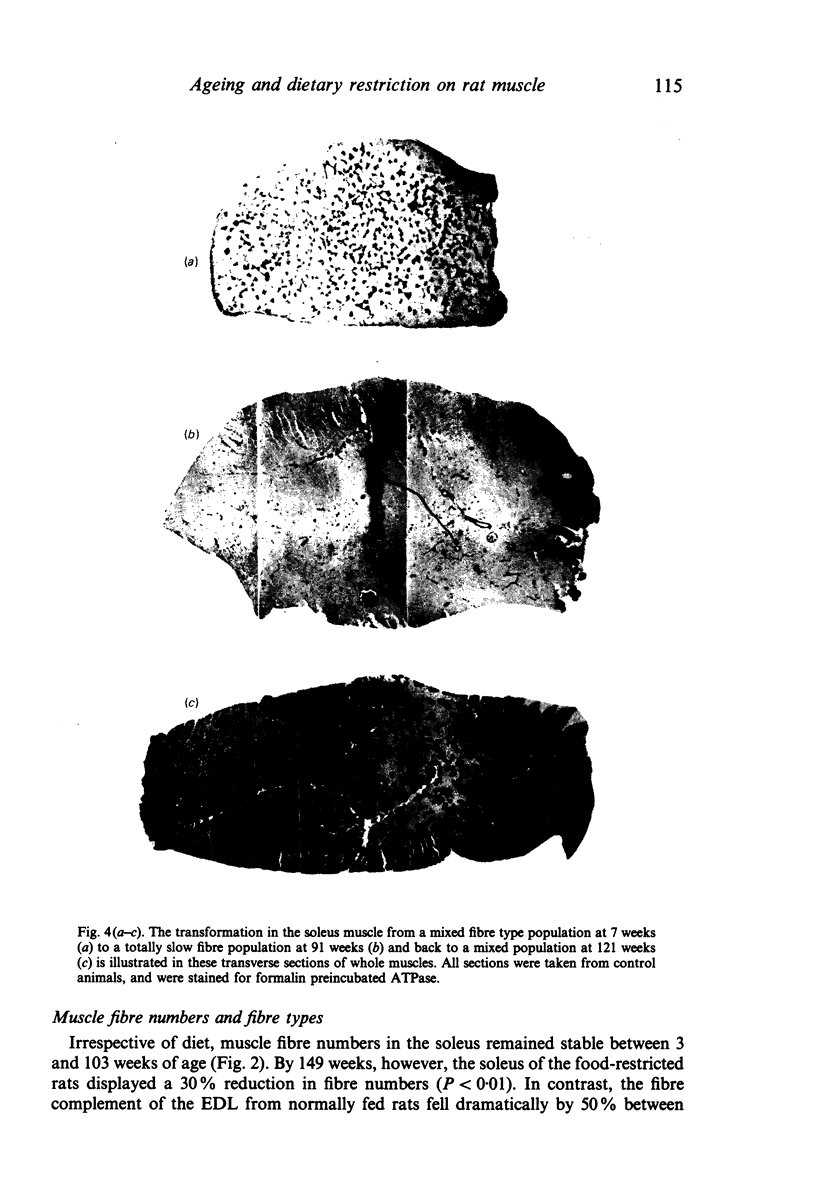
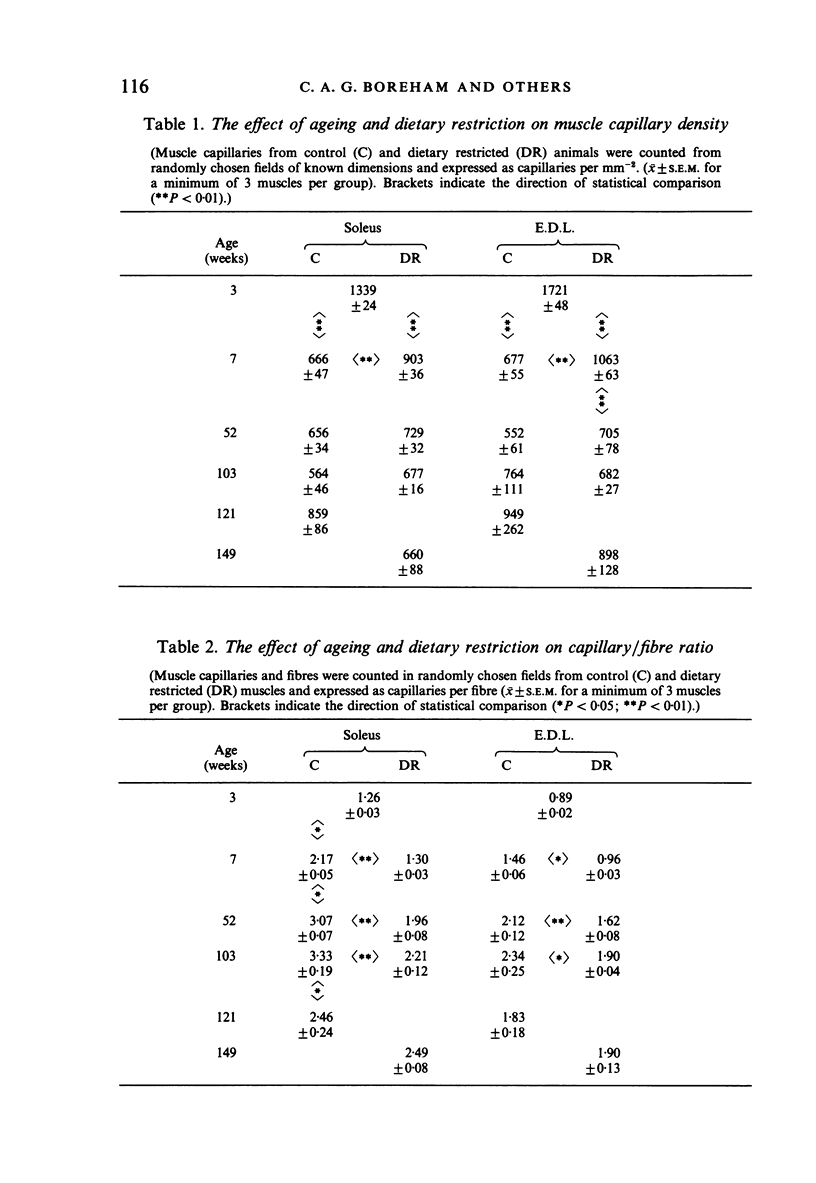
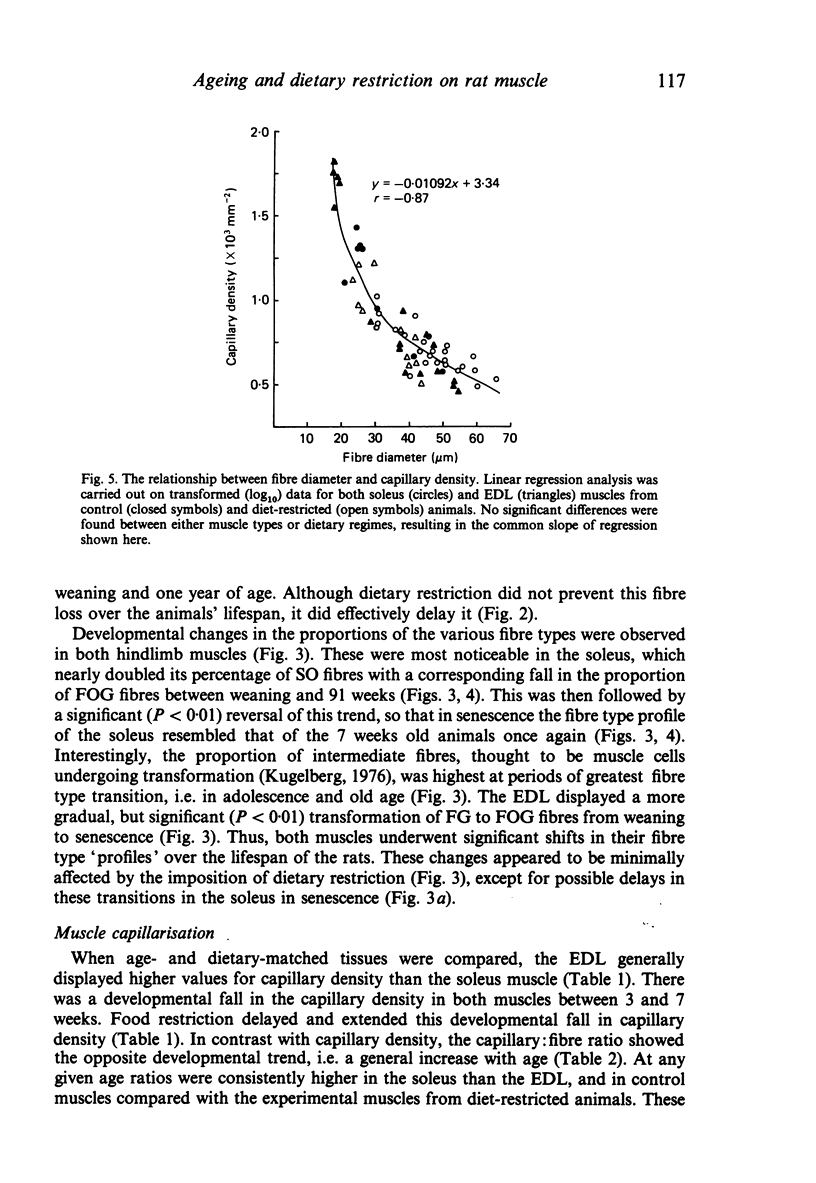
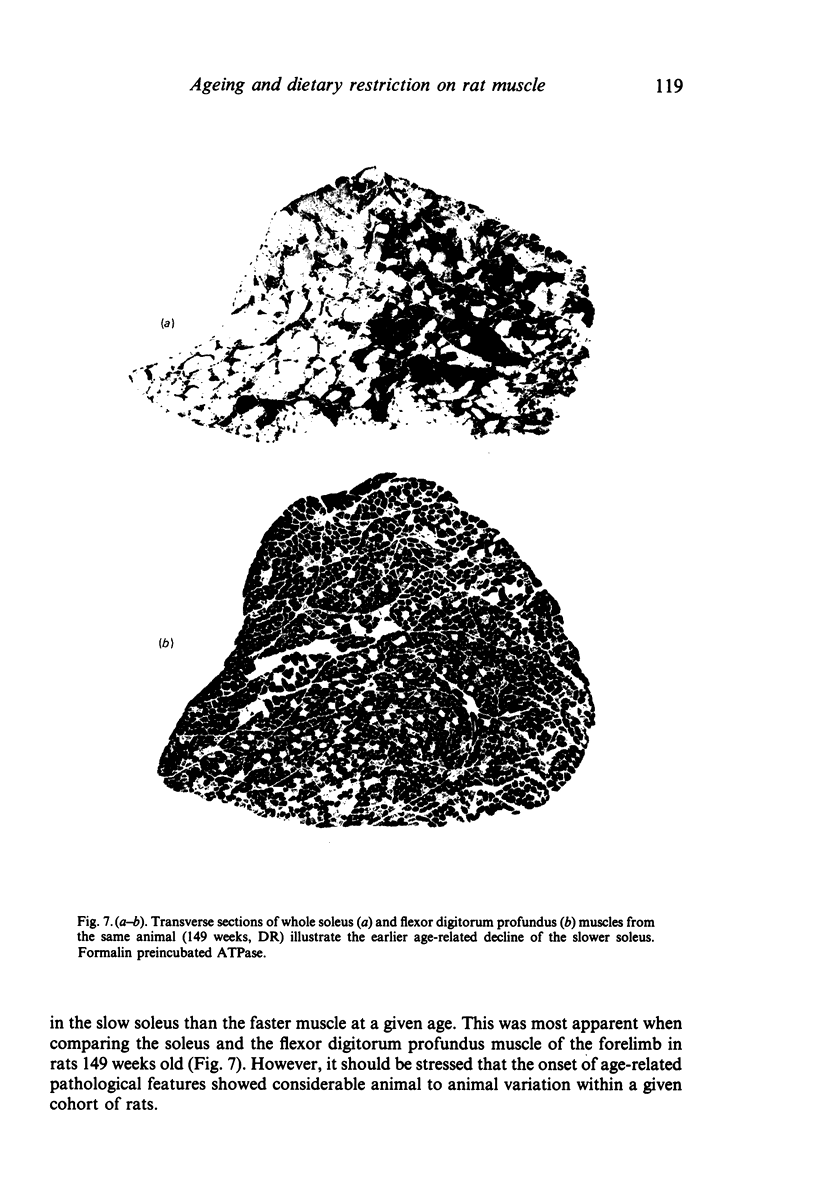
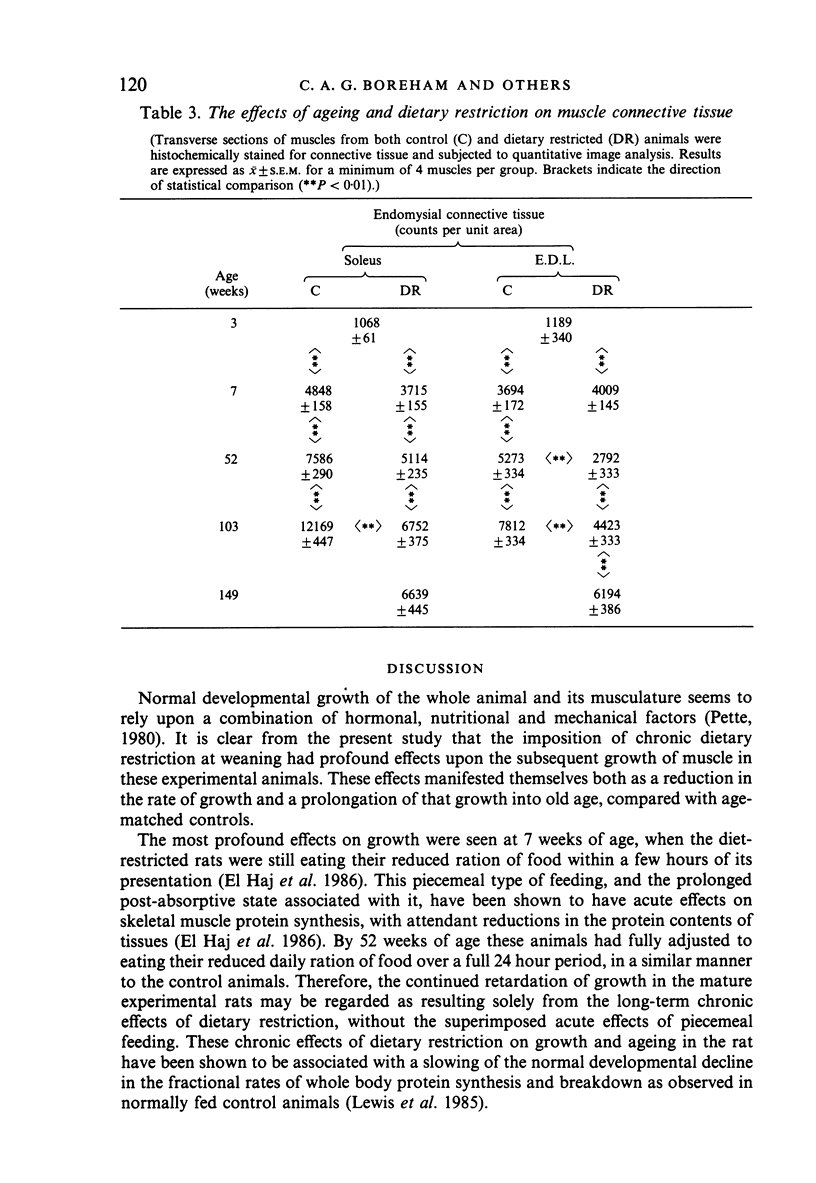
The soleus and extensor digitorum longus muscles of the hindlimb and the flexor digitorum profundus muscle of the forelimb were studied in ad libitum-fed control and age-matched diet-restricted male rats at various ages from weaning to senescence. Growth of individual muscles was accomplished by fibre hypertrophy and not hyperplasia. Between weaning and one year, fibre numbers remained constant in the soleus but fell by 50% in the extensor digitorum longus. Both muscles displayed increasingly oxidative fibre type profiles with advancing age, irrespective of dietary status. This was particularly noticeable in the soleus, which transformed its fibre population from one containing 35% fast fibres at weaning to one with no fast fibres at 91 weeks. In senility, however, the fibre type population again displayed 25% fast fibres. The capillary: fibre ratio and the capillary density were correlated with muscle fibre size in both hindlimb muscles. Although capillarity increased with age, expected differences between fast and slow muscles were probably minimised by the high proportion of FOG fibres in the extensor digitorum longus. Both hindlimb muscles displayed significant increases in the ratio of connective: muscle tissue with increasing age. The soleus invariably contained more connective tissue than the extensor digitorum longus. Dietary restriction reduced the rate of increase, so that the connective tissue content was approximately one half that found in control muscles at one year. Various pathological features associated with old age were delayed considerably in the muscles of the diet-restricted rats. It is concluded that chronic dietary restriction imposed directly after weaning has a dramatic effect on the normal growth and ageing of skeletal muscle.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquin L., Sillau A. H., Lechner A. J., Banchero N. Growth and skeletal muscle microvascularity in the guinea pig. Microvasc Res. 1980 Jul;20(1):41–50. doi: 10.1016/0026-2862(80)90018-7. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Kelso J. R. Effect of hind-limb immobilization on contractile and histochemical properties of skeletal muscle. Pflugers Arch. 1973 Aug 27;342(3):231–238. doi: 10.1007/BF00591371. [DOI] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Constantine V. S. A combined tissue stain for the selective staining of collagen, elastic fibers and acidic carbohydrates. J Invest Dermatol. 1969 Apr;52(4):353–356. doi: 10.1038/jid.1969.60. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essén-Gustavsson B., Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986 Jan;126(1):107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Lewis S. E., Merry B. J. Effects of aging and long term dietary intervention on protein turnover and growth of ventricular muscle in the rat heart. Cardiovasc Res. 1986 Sep;20(9):672–678. doi: 10.1093/cvr/20.9.672. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Timson B. F., Moore R. L., Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol Respir Environ Exerc Physiol. 1981 May;50(5):936–943. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- Gray S. D., Renkin E. M. Microvascular supply in relation to fiber metabolic type in mixed skeletal muscles on rabbits. Microvasc Res. 1978 Nov;16(3):406–425. doi: 10.1016/0026-2862(78)90073-0. [DOI] [PubMed] [Google Scholar]
- Grimby G., Saltin B. The ageing muscle. Clin Physiol. 1983 Jun;3(3):209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Kovanen V., Suominen H., Heikkinen E. Connective tissue of "fast" and "slow" skeletal muscle in rats--effects of endurance training. Acta Physiol Scand. 1980 Feb;108(2):173–180. doi: 10.1111/j.1748-1716.1980.tb06515.x. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Layman D. K., Hegarty P. V., Swan P. B. Comparison of morphological and biochemical parameters of growth in rat skeletal muscles. J Anat. 1980 Jan;130(Pt 1):159–171. [PMC free article] [PubMed] [Google Scholar]
- Lewis S. E., Goldspink D. F., Phillips J. G., Merry B. J., Holehan A. M. The effects of aging and chronic dietary restriction on whole body growth and protein turnover in the rat. Exp Gerontol. 1985;20(5):253–263. doi: 10.1016/0531-5565(85)90050-6. [DOI] [PubMed] [Google Scholar]
- Martin W. D., Romond E. H. Effects of chronic rotation and hypergravity on muscle fibers of soleus and plantaris muscles of the rat. Exp Neurol. 1975 Dec;49(3):758–771. doi: 10.1016/0014-4886(75)90057-6. [DOI] [PubMed] [Google Scholar]
- Merry B. J., Holehan A. M. The endocrine response to dietary restriction in the rat. Basic Life Sci. 1985;35:117–141. doi: 10.1007/978-1-4899-2218-2_6. [DOI] [PubMed] [Google Scholar]
- Mohan S., Radha E. Age-related changes in rat muscle collagen. Gerontology. 1980;26(2):61–67. doi: 10.1159/000212396. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Salminen A., Peltonen L., Takala T. E., Vihko V. Collagen metabolism of mouse skeletal muscle during the repair of exercise injuries. Pflugers Arch. 1986 Jul;407(1):64–70. doi: 10.1007/BF00580722. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Plyley M. J., Groom A. C. Geometrical distribution of capillaries in mammalian striated muscle. Am J Physiol. 1975 May;228(5):1376–1383. doi: 10.1152/ajplegacy.1975.228.5.1376. [DOI] [PubMed] [Google Scholar]
- Stebbins C. L., Schultz E., Smith R. T., Smith E. L. Effects of chronic exercise during aging on muscle and end-plate morphology in rats. J Appl Physiol (1985) 1985 Jan;58(1):45–51. doi: 10.1152/jappl.1985.58.1.45. [DOI] [PubMed] [Google Scholar]
- Templeton G. H., Padalino M., Manton J., Glasberg M., Silver C. J., Silver P., DeMartino G., Leconey T., Klug G., Hagler H. Influence of suspension hypokinesia on rat soleus muscle. J Appl Physiol Respir Environ Exerc Physiol. 1984 Feb;56(2):278–286. doi: 10.1152/jappl.1984.56.2.278. [DOI] [PubMed] [Google Scholar]
- Tunell G. L., Hart M. N. Simultaneous determination of skeletal muscle fiber, types I, IIA, and IIB by histochemistry. Arch Neurol. 1977 Mar;34(3):171–173. doi: 10.1001/archneur.1977.00500150057011. [DOI] [PubMed] [Google Scholar]
- Watt P. W., Kelly F. J., Goldspink D. F., Goldspink G. Exercise-induced morphological and biochemical changes in skeletal muscles of the rat. J Appl Physiol Respir Environ Exerc Physiol. 1982 Nov;53(5):1144–1151. doi: 10.1152/jappl.1982.53.5.1144. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Connective tissue changes in immobilised muscle. J Anat. 1984 Mar;138(Pt 2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Connective tissue changes in surgically overloaded muscle. Cell Tissue Res. 1981;221(2):465–470. doi: 10.1007/BF00216749. [DOI] [PubMed] [Google Scholar]
- Ziada A. M., Hudlicka O., Tyler K. R., Wright A. J. The effect of long-term vasodilatation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res. 1984 Dec;18(12):724–732. doi: 10.1093/cvr/18.12.724. [DOI] [PubMed] [Google Scholar]
- el Haj A. J., Lewis S. E., Goldspink D. F., Merry B. J., Holehan A. M. The effect of chronic and acute dietary restriction on the growth and protein turnover of fast and slow types of rat skeletal muscle. Comp Biochem Physiol A Comp Physiol. 1986;85(2):281–287. doi: 10.1016/0300-9629(86)90251-3. [DOI] [PubMed] [Google Scholar]




