Abstract
Shigellosis is an acute inflammatory bowel disease caused by the enteroinvasive bacterium Shigella. Upon host cell–Shigella interaction, major host cell signalling responses are activated. Deciphering the initial molecular events is crucial to understanding the infectious process. We identified a molecular complex involving proteins of both the host, CD44 the hyaluronan receptor, and Shigella, the invasin IpaB, which partitions during infection within specialized membrane microdomains enriched in cholesterol and sphingolipids, called rafts. We also document accumulation of cholesterol and raft-associated proteins at Shigella entry foci. Moreover, we report that Shigella entry is impaired after cholesterol depletion using methyl-β-cyclodextrin. Finally, we find that Shigella is less invasive in sphingosid-based lipid-deficient cell lines, demonstrating the involvement of sphingolipids. Our results show that rafts are implicated in Shigella binding and entry, suggesting that raft-associated molecular machineries are engaged in mediating the cell signalling response required for the invasion process.
Keywords: CD44/cholesterol/IpaB/Shigella/sphingolipid rafts
Introduction
Shigella is a Gram-negative enteroinvasive bacterium known as the causative agent of bacillary dysentery (Sansonetti, 2001; Suzuki and Sasakawa, 2001). Shigellosis is endemic throughout the world, although most of the million yearly deaths, 70% of which affect children between the ages of 1 and 5 years, occur in developing countries. After oral contamination, the pathogen reaches the colon where it is translocated across the epithelial barrier by the M cells in the Peyer patches. Once translocated, Shigella infect macrophages in which they trigger cell death. As a result, polymorphonuclear cells are activated and transmigrate through the epithelium via a process called ‘fatal attraction’, thus disrupting the monolayer and allowing rapid basolateral Shigella invasion of epithelial cells. This ‘snowball effect’ of inflammation and invasion induces severe inflammatory destruction, characteristic of the disease (Sansonetti, 2001). To be virulent, Shigella isolates carry a plasmid encoding the ‘invasive phenotype’ of the particular species. The pWR100 (214 kb) plasmid of the M90T strain of Shigella flexneri 5a has been fully sequenced. It contains a ‘pathogenicity island’ (30 kb) encompassing the ipa/mxi–spa operons, which encode the proteins composing the type III secretion system, or secreton, and effector proteins. The secreton is evolutionarily related to the flagellar origin but is used as a needle that injects effector proteins into the host cell cytoplasm (Blocker et al., 2001). Among these effectors, the Ipa proteins play essential roles: IpaB and IpaD form a complex that controls the flux of proteins through the secreton, while IpaB and IpaC insert into the plasma membrane of the host cell to form a pore. In addition, IpaA and IpaC are involved in actin polymerization and bundling, whereas an IpaB–IpaC complex is needed for the bacterium to break the phagocytic vacuole once the bacterium is inside the host cell (Sansonetti, 2001). In monocyte/macrophage and dendritic populations, IpaB additionally binds to caspase I, leading to apoptosis with release of the pro-inflammatory molecules IL-1β and IL-18 (Sansonetti, 2001).
Using several biochemical assays, IpaB was shown to bind the transmembrane receptor CD44 (Skoudy et al., 2000). CD44 is a member of the immunoglobulin superfamily that binds hyaluronan. CD44 has been reported to bind via its cytoplasmic tail the ezrin–radixin–moesin (ERM) family of proteins that associate with the actin cytoskeleton (Hirao et al., 1996). Ezrin was shown to be recruited at the Shigella entry structure and to be required for the entry process (Skoudy et al., 1999). CD44 may therefore help to regulate the actin assembly/disassembly that occurrs during Shigella entry, as the CD44–ERM complex activates RhoGDI, a negative regulator of Rho proteins that were found to be implicated in Shigella entry (Watarai et al., 1997; Mounier et al., 1999). Alternatively, CD44 could be involved in the adhesion of the secreton to the host cell surface. Identical mechanisms can be proposed for the reported interaction between IpaB and the β1 integrin (Watarai et al., 1996). Since IpaC and IpaD mutants are totally deficient for invasion despite the fact that they secrete IpaB (Parsot et al., 1995), it is likely that this CD44–IpaB interaction is implicated in a discrete step of the entry process. This step, however, conditions the action of other Shigella effectors.
CD44 distributes along the basolateral membrane of mammary epithelial cell lines where it partitions within specialized lipid microdomains (Oliferenko et al., 1999). These microdomains, also called rafts, are cholesterol/sphingolipid-enriched membranes involved in cell signalling, membrane trafficking and cholesterol metabolism, and are proposed to play an important role in several diseases (Simons and Ikonen, 1997; Brown and London, 1998). An important feature of rafts is their highly dynamic nature, which allows the transient formation of membrane platforms that build up molecular machineries implicated in cell signalling or targeting mechanisms. Microbiologists became interested in rafts because they provide an ideal clustering device that could trigger cell signalling events required for pathogen–host cell interaction and/or entry (Fivaz et al., 1999; Rosenberger et al., 2000). Several pathogens were indeed shown to interact specifically with raft assemblies at the cell surface. For example, viruses such as SV40 are internalized into the host cell via caveolae that are specialized, flask-shaped, rafts found at the cell surface and involved in endocytosis and cell signalling (Kartenbeck et al., 1989; Anderson et al., 1996; Stang et al., 1997). Also, several bacteria were described as entering host cells in a cholesterol-dependent manner (Fivaz et al., 1999; Rosenberger et al., 2000). It is also worth noting that a variety of bacterial toxins were shown to bind raft-associated molecules (Fivaz et al., 1999).
In this study, using several approaches, we have asked the questions of whether IpaB–CD44 would be present within raft domains during infection and whether Shigella is using a cholesterol/sphingolipid-mediated pathway to bind and enter the host cell.
Results
IpaB and CD44 co-fractionate with detergent-resistant membranes
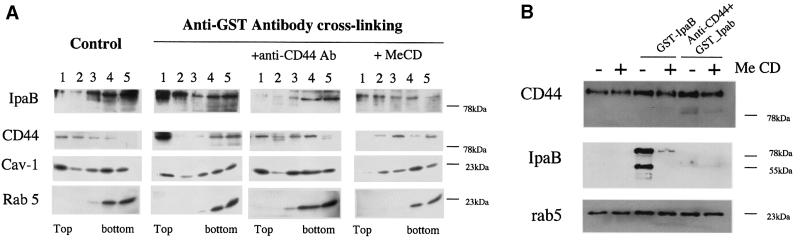
In order to address the role of lipid rafts in the IpaB–CD44 interaction during Shigella infection, we first analysed whether recombinant IpaB fused to glutathione S-transferase (rec GST–IpaB) would associate with lipid rafts, assayed here by detergent insolubility, a method widely accepted for investigation of the raft association of a given protein (Simons and Toomre, 2000). HeLa cells were incubated with rec GST–IpaB for 1 h at 11°C to prevent internalization while maintaining membrane fluidity. Samples were scraped and treated with Triton X-100 on ice for 30 min. Detergent-resistant membranes (DRMs) were subsequently purified by flotation on OptiPrep step gradients. As shown in Figure 1A, rec GST–IpaB was found mainly in the detergent-soluble fractions at the bottom of the gradient, co-distributed with the small GTPase rab5, which was used here as a non-raft marker. In contrast, CD44 was found in the lighter fractions of the gradient, in agreement with previous observations (Oliferenko et al., 1999), together with caveolin-1 (Cav-1), a well-known marker of DRMs. Since it has been shown previously by Huber and collaborators (Oliferenko et al., 1999) that all CD44 isoforms are raft associated, we have herein followed the most prominent form, CD44s (mol. wt ∼85 kDa). Since antibody cross-linking of raft components has been shown previously to induce the formation of raft patches at the cell surface (Harder et al., 1998), we tested in parallel experiments, whether we could thus induce raft association of IpaB. When surface-bound GST–IpaB was cross-linked by incubating the cells sequentially with anti-GST and horse radish peroxidase (HRP)-conjugated secondary antibodies, both rec GST–IpaB and CD44 were found mainly in the top DRM fraction. Such redistribution after antibody cross-linking was not observed when rec GST alone was added to the growth medium of HeLa cells (not shown), indicating that the redistribution of IpaB and CD44 was not due to the GST tag. The redistribution of IpaB to the DRM fractions upon cross-linking was CD44 mediated, since incubation with anti-CD44 antibodies before and during the incubation with rec GST–IpaB prevented IpaB from associating with DRMs. The redistribution of IpaB to the DRMs upon cross-linking was reduced by extracting membrane cholesterol with methyl-β-cyclodextrin (MeCD), a treatment known to disorganize raft microdomains and which also led to the redistribution of CD44 to detergent-soluble fractions.
Fig. 1. (A) Recombinant GST–IpaB and CD44 co-fractionate with DRMs upon antibody cross-linking. HeLa cells were incubated with GST–IpaB and scraped. Membranes were extracted with 0.5% Triton X-100 for 30 min on ice and subsequently flotation in a step density gradient was performed. Fractions were collected from the top and proteins were precipitated and analysed by western blotting. For clustering experiments, cells were incubated sequentially with rec GST–IpaB, anti-GST antibodies and GAM–TRITC antibodies. For competition experiments, cells were incubated with the anti-CD44 antibody before and during the incubation with rec GST–IpaB. For cholesterol depletion, cells were treated with MeCD prior to incubation with rec GST–IpaB. All incubations were performed for 1 h at 11°C separated by three washes. One representative experiment of four is shown. (B) IpaB binding is reduced by MeCD treatment. HeLa cells treated or not with MeCD were incubated for 1 h at 11°C with rec GST–IpaB or with anti-CD44 antibody plus rec GST–IpaB. Aliquots of cell extracts were analysed by western blotting. The low molecular weight bands revealed by the anti-IpaB antibody correspond to degradative products of the GST–IpaB fusion protein (see Materials and methods).
In addition to reducing the raft association of IpaB (Figure 1A), MeCD treatment appeared to diminish binding of IpaB to cells. To verify this point, we analysed by western blotting of total cell extracts the binding of IpaB to HeLa cells under various conditions. As shown in Figure 1B, after treating cells with MeCD, binding of IpaB was significantly reduced, despite the fact that the levels of CD44 were the same as in control cells. Inhibition of IpaB binding was, however, less dramatic than that observed after pre-incubation of the cells with anti-CD44 antibodies. Rab5 was used as an equal loading marker.
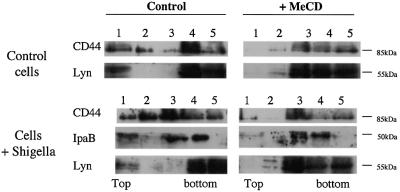
The above observations show that recombinant IpaB is able to associate with DRMs with high efficiency providing it is clustered with antibodies. Antibody clustering could mimic a physiological situation occurring during infection, where several IpaB molecules, associated with multiple secretons on the bacterium, would interact with the target cell surface. We therefore investigated whether IpaB was also associated with DRMs during infection. HeLa cells were challenged with Shigella. Samples were solubilized in Triton X-100 and DRMs were isolated on OptiPrep gradients. As shown in Figure 2, IpaB was found mainly associated with DRMs. Infection also led to increased association of CD44 with DRMs. DRM association of both IpaB and CD44 was prevented by MeCD treatment of the cells, as shown in Figure 1. The Src-like kinase Lyn, a pool of which is associated with DRMs in a cholesterol-dependent manner, was used as positive control.
Fig. 2. IpaB and CD44 associate with DRMs upon infection. HeLa cells infected or not with the S.flexneri M90T strain were scraped and membranes extracted with 0.5% Triton X-100 for 1 h on ice before flotation. Fractions were collected from the top and proteins precipitated before analysis by western blotting.
Cholesterol involvement during Shigella infection
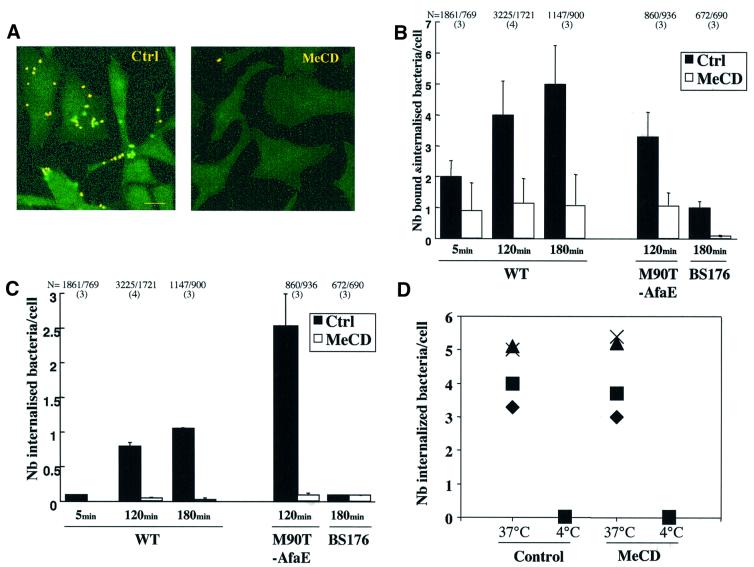
The above observations clearly show that the Shigella protein IpaB associates with lipid rafts during infection, raising the possibility that these plasma membrane domains are preferential host cell interaction sites during infection. To address this issue, we investigated whether MeCD treatment, which prevents DRM association of IpaB, affects Shigella infection. HeLa cells were pre-treated or not with MeCD, infected with Shigella and then fixed. Cell-associated bacteria were detected using anti-lipopolysaccharide (LPS) antibodies. Internalized bacteria were detected by inside-out immunostaining. To distinguish bound from internalized bacteria, fixed cells were first incubated with a monoclonal anti-LPS antibody, then permeabilized and incubated with a polyclonal anti-LPS antibody. After addition of TRITC anti-mouse and FITC anti-rabbit secondary antibodies, internalized bacteria appeared green whereas surface-bound bacteria appeared yellow, having bound both the mono- and polyclonal anti-LPS antibodies (Skoudy et al., 1999), as shown in a typical experiment in Figure 3A. When similar experiments were performed on MeCD-treated cells, very few cell-associated bacteria could be detected. Quantifications showed that after 180 min, whereas 5.0 ± 1.25 bacteria were bound and internalized per control cell, only 1.0 ± 1.05 bacteria were bound and internalized per MeCD-treated cell (Figure 3B). This corresponds to the background levels observed with the non-invasive BS176 Shigella strain, which has been cured of the virulent plasmid (Sansonetti et al., 1982) (Figure 3B). To investigate the role of rafts further in the entry process, we measured binding and entry of another virulent Shigella strain that expresses the adhesive fimbrial AfaE protein from Escherichia coli (M90T-AfaE; Garcia et al., 1994), which binds the GPI-anchored protein CD55 (Nowicki et al., 1993). This strain binds the host cell with high efficiency, explaining why a low bacterial titre was used to get a binding yield comparable to that of the wild-type strain (see Materials and methods). After 120 min, whereas 3.3 ± 0.8 M90T-AfaE bacteria were bound and internalized per control cell, only 1.0 ± 0.2 bacteria were bound and internalized per MeCD-treated cell (Figure 3B). Since binding of M90T-AfaE after MeCD was still significant, albeit reduced, we quantified the number of intracellular bacteria. Whereas 2.5 ± 0.5 intracellular bacteria were counted per control cell, background levels (0.08 ± 0.01 bacteria/cell) were observed after MeCD treatment (Figure 3C). Together these results show that cholesterol depletion affects both Shigella binding and entry into the host cell.
Fig. 3. Cholesterol depletion impairs S.flexneri infectivity. (A) Cells were treated or not with 10 mM MeCD (1 h at 37°C) and infected with the M90T strain. Samples were fixed and processed for immunocytochemistry to label bound and internalized bacteria as described in Materials and methods. Bacteria bound to the cell surface appear yellow and internalized bacteria green. Bar = 15 µm. (B) HeLa cells infected with S.flexneri M90T strain were processed for immunostaining as in (A), and bound bacteria were counted under the microscope. N indicates the total number of cells analysed and the number in parentheses the number of independent experiments analysed. (C) Internalized bacteria corresponding to the same experiments as shown in (B). (D) HeLa cells were infected at 37 or 4°C with a strain of E.coli (YInv+). When required, cells were treated with 10 mM MeCD for 1 h at 37°C before infection. After a 1 h infection, sample was fixed and bacteria were identified by immunolabelling using an anti-LPS antibody. Results of four independent experiments are shown in which 106–601 cells were analysed per experiment.
We wanted to ascertain that the treatment with MeCD was not affecting the membrane such that no pathogen whatsoever could bind and hence be internalized. Thus, we measured binding and entry of an E.coli strain expressing the invasin from Yersinia [E.coli (YInv+)] into HeLa cells. As shown in Figure 3D, in four independent experiments, the same internalization efficiency was observed whether or not cells had been treated with MeCD. As a negative control, infection was performed at 4°C.
Cholesterol and raft-associated proteins accumulate at Shigella entry foci
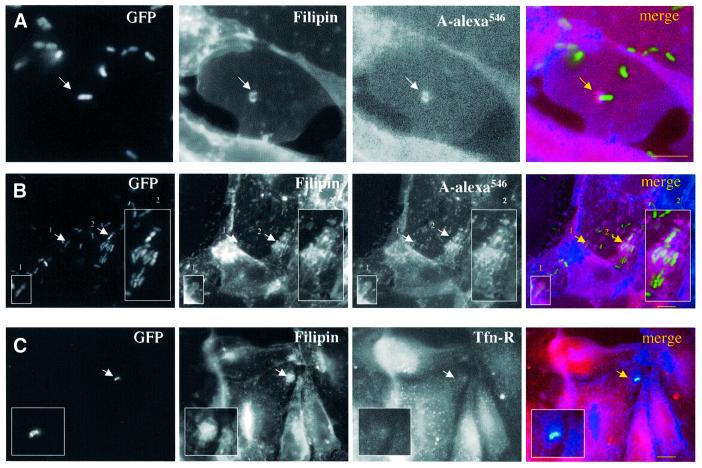
The above quantitative data pointed towards an important role played by cholesterol during infection. We therefore investigated whether we could visualize the presence of cholesterol at Shigella entry points and during the early invasion period, when the bacterium has not yet escaped from the phagocytic vacuole. Experiments were carried out within a precise short time range, since bacteria are rapidly internalized (15 min in average) and escape the vacuolar compartment within the first 15 min after internalization (Sansonetti et al., 1986). HeLa cells were therefore infected with green fluorescent protein (GFP)-tagged Shigella for 15 min at 37°C, fixed and processed for fluorescence microscopy (Figure 4). Cholesterol distribution was visualized with filipin, a fluorescent probe that binds cholesterol. High staining for cholesterol was clearly observed at the entry foci (Figure 4A) and vacuolar membranes (Figure 4B). Interestingly, similar cholesterol staining was observed recently in the entry site of Salmonella (Catron et al., 2002; Garner et al., 2002). Since Shigella triggers the formation of membrane ruffles at the point of entry, this apparent accumulation of cholesterol could merely represent an increase in local membrane surface rather than a bona fide increase in cholesterol. To address this issue we performed labelling against two surface markers: GPI-anchored proteins were labelled using the GPI-specific probe aerolysin labelled with Alexa546 (PA-Alexa546; Fivaz et al., 2002), and the transferrin receptor (Tfn-R) was labelled with specific antibodies. As shown by the arrows in Figure 4, accumulation of GPI-anchored protein was observed at the entry foci and on vacuolar membranes (Figure 4A, arrow and B, inserts). In contrast, we did not observe an increase in Tfn-R labelling at the entry foci (Figure 4C, insert). Since GPI-anchored proteins are well-known raft components whereas, the Tfn-R is the prototype of a non-raft protein (Simons and Ikonen, 1997; Brown and London, 1998), these observations suggest that raft components, including cholesterol and endogenous GPI-anchored proteins, accumulate in the vicinity of Shigella entry points.
Fig. 4. Cholesterol accumulates at S.flexneri entry foci. HeLa cells were infected with GFP-tagged S.flexneri M90T strain for 15 min at 37°C, fixed and processed for labelling. Cholesterol distribution was analysed with filipin. (A and B) GPI-anchored proteins were labelled with aerolysin ASSP-Alexa546 conjugated (A-alexa546). (C) Transferrin receptor (Tfn-R) was visualized after immunostaining using a specific antibody followed by a TRITC-conjugated secondary antibody. Bar = 10 µm.
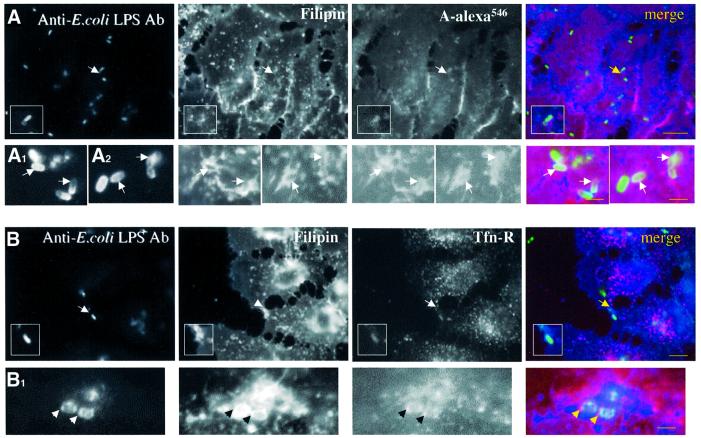
As a negative control for these morphological studies we analysed the distribution of cholesterol, GPI-anchored proteins and the Tfn-R at the early time points of infection of E.coli harbouring the Yersinia invasin (YInv+; Figure 5) since we have shown in Figure 3D that infection with this strain was not affected by MeCD. Bacterial distribution was followed by immunocytochemistry on non-permeabilized cells. Therefore, internalized bacteria did not always appear homogeneously labelled due to lower efficiency in staining procedure. For example, two bacteria are shown in the insert of Figure 5, the bottom one being internalized. This internalized bacterium was enclosed within membranes positive for both filipin and PA-Alexa546. Other examples are shown in Figure 5A1 and A2. The vacuolar membrane was also positive for Tfn-R as shown in Figure 5B and B1, suggesting that, in contrast to Shigella, E.coli (YInv+) was engulfed within a vacuole that shows no selectivity towards raft and non-raft domains.
Fig. 5. Non-selective internalization of plasma membrane proteins during E.coli (YInv+) invasion. HeLa cells were infected with E.coli (YInv+) for 20 min at 37°C, fixed and processed for labelling. Bacteria were visualized using anti-E.coli LPS antibody and Alexa488-conjugated secondary antibodies. Cholesterol distribution was analysed with filipin. (A) GPI-anchored proteins were labelled with aerolysin ASSP-Alexa546 conjugated (A-alexa546). In (A1) and (A2) other examples of bacterial internalization are shown at higher magnification. (B and B1) Tfn-R was visualized (B1: high magnification) after immunostaining using a specific antibody followed by a TRITC-conjugated secondary antibody. Bar for (A) and (B) = 10 µm; (A1) and (B1) = 3 µm.
Raft involvement in Shigella entry
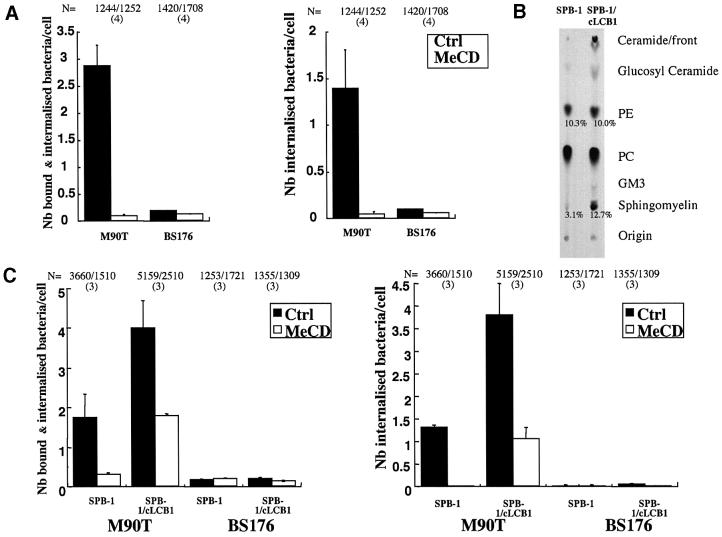
Lipid raft microdomains are enriched both in cholesterol and sphingolipids. Having shown the importance of cholesterol in Shigella entry, we next analysed the requirement for sphingolipids. Rather than treating cells with drugs such as fumonisin, we opted for cells deficient for the synthesis of sphingoid-based lipids. As this mutant cell line is derived from Chinese hamster ovary (CHO.K1) cells, we first checked whether wild- type CHO cells were susceptible to Shigella infection. As shown in Figure 6A, CHO cells were indeed susceptible to infection by Shigella and importantly, as for HeLa cells, binding and internalization were inhibited to almost background levels (BS176 non- invasive strain) by MeCD pre-treatment. It is important to note that CD44 is expressed in CHO and associates with DRMs as shown for HeLa cells (F.Lafont, unpublished results). We next compared the susceptibility of sphingolipid-deficient CHO cells (SPB-1) to the corresponding recomplemented cell line (SPB-1/cLCB1). SPB-1 cells are deficient in sphingosid-based biosynthesis (Hanada et al., 1990) due to a temperature-sensitive mutation in the serine palmitoyltransferase (SPT), the enzyme that catalyses the first step in the sphingolipid biosynthetic pathway, in which l-serine condenses with palmitoyl-CoA to produce 3-ketodihydrosphingosine. SPT activity in SPB-1 cells can be restored by stable transfection with the cLCB1 gene corresponding to the CHO cDNA homologue of the yeast LCB1 gene (Hanada et al., 1997). As determined by quantitative TLC analysis (Figure 6B), sphingomyelin and ceramide levels were drastically reduced in SPB-1 cells as compared with the recomplemented SPB-1/cLCB1, in agreement with previous results (Hanada et al., 1990).
Fig. 6. Sphingolipids are required for efficient entry of S.flexneri into the host cell. (A) CHO.K1 cells were pre-treated or not with 10 mM MeCD for 1 h at 37°C before infection with S.flexneri invasive (M90T) or non-invasive (BS176) strain for 20 min at 37°C. Bound and internalized bacteria were identified by immunolabelling as described in Materials and methods. N indicates the total number of cells analysed and the number in parentheses the number of independent experiments analysed. (B) TLC analysis of lipids in sphingosid-based lipid-deficient CHO cell line (SPB-1) and recomplemented cells (SPB-1/cLCB1). Percentages are given versus total lipids. Note the lower amount of sphingomyelin and ceramide in the SPB-1 cells versus the SPB-1/cLCB1 cells. (C) SPB-1 and SPB-1/cLCB1 cells were pre-treated or not with 10 mM MeCD for 1 h at 37°C before being infected with the S.flexneri M90T or BS176 strain for 60 min at 37°C. N indicates the total number of cells analysed and the number in parentheses the number of independent experiments analysed.
As quantified in Figure 6C, SPB-1 cells showed significantly reduced invasion by Shigella when compared with SPB-1/cLCB1 cells, highlighting a role of sphingolipids in the infectious process. Invasion was not completely inhibited probably due to the fact that SPB-1 cells still contain lipid rafts/DRMs, although in lower amounts than control cells (Hanada et al., 1990). We therefore investigated the effect of MeCD on the invasion of SPB-1 cells. As shown in Figure 6C, MeCD treatment further reduced Shigella invasion to background levels (BS176 strain). Similar results were obtained with the invasive M90T-AfaE strain expressing the AfaE adhesin versus the same strain with the mxiD gene inactivated used as control (F.Lafont, unpublished results).
Discussion
In this study, we report biochemical, morphological and functional evidence demonstrating the importance of plasma membrane cholesterol and sphingolipids in the very first steps of Shigella–host cell interactions, i.e. binding and internalization. We provide the first biochemical demonstration that a molecular complex formed by an effector protein from the pathogen, IpaB, and a transmembrane protein from the host, CD44, partitions within rafts during infection. This was shown by analysing binding of both recombinant IpaB and the bacterium. Importantly, CD44 is known to participate in signalling responses regulating cytoskeleton organization (Hirao et al., 1996). Interestingly, the integrity of rafts seems to be required for efficient binding of IpaB to the host cells, presumably because CD44 must be raft associated. This could be due to various reasons: (i) CD44 might change its conformation upon raft association and become competent for IpaB binding; (ii) additional molecules present in rafts could increase the stability of the CD44–IpaB interaction; (iii) finally, as discussed below, binding of multiple IpaB molecules to clustered CD44 could be crucial for efficient adherence of the bacterium to the cell. Clearly, these results apply to cells expressing CD44, such as epithelial cells of the digestive tract (Neame and Isacke, 1993). On other cells, the interaction of the IpaB–IpaC complex with the α5β1 integrin (Watarai et al., 1996) could also involve rafts, since association of integrins with raft domains has been reported (Wary et al., 1998).
The physiological relevance of the raft association of the IpaB–CD44 complex is strongly supported by the observation that cholesterol extraction dramatically impairs binding and internalization of Shigella. The association of Shigella with specific membrane domains is reminiscent of the recruitment of CD44 in the vicinity of the bacterial entry foci as observed by immunofluorescence and electron microscopy (Skoudy et al., 2000). To confirm the involvement of rafts rather than other types of microdomain, we showed that GPI-anchored proteins [well known to associate with rafts (Simons and Ikonen, 1997; Brown and London, 1998)], in addition to cholesterol, accumulated at the sites of Shigella entry, whereas the Tfn-R did not. Since the driving force for lipid raft formation is thought to be the lateral aggregation of cholesterol and sphingolipids, we further evaluated the effect of sphingolipid depletion on Shigella entry using a cell line deficient in sphingolipid biosynthesis. Infection of these cells was significantly impaired and once more appeared to be cholesterol dependent. Hence, our observations demonstrate the involvement of lipid raft microdomains in the multifactorial trigger mechanism described for Shigella infection (Sansonetti, 2001). Involvement of rafts in bacterial entry mechanisms has been proposed for some E.coli strains (Baorto et al., 1997; Guignot et al., 2000) that enter via caveolae, as well as for Myco bacterium spp. (Gatfield and Pieters, 2000), Chlamydia trachomatis (Norkin et al., 2001) and Salmonella, which has type III secretion machineries similar to that of Shigella (Garner et al., 2002). In these latter cases, raft involvement was, however, almost exclusively based on cholesterol depletion experiments.
Shigella flexneri is engulfed by a macropinocytic process (Clerc and Sansonetti, 1987; Adam et al., 1995) and this is therefore unlikely to be caveolae mediated as proposed for adhesin-expressing E.coli (Baorto et al., 1997; Guignot et al., 2001). It is important to emphasize that S.flexneri does not adhere strongly to the cell surface, as opposed to E.coli expressing fimbrial adhesive proteins, which is why, in vitro, contact must always be promoted by spinning the bacteria on to the cell surface. The reasons why these two types of bacterium require lipid rafts for efficient invasion could therefore be very different. One could speculate that E.coli binds in a raft-independent manner but then triggers patching of raft domains and de novo caveolae formation around the bacterium, a process required for internalization. In contrast, rafts, and thereby clustering of receptor molecules, would be crucial for efficient S.flexneri binding, since it does not express adhesins. Simultaneous binding of multiple IpaB, each at the tip of a secreton, to clustered CD44 molecules is indeed likely to increase binding affinity. In addition to promoting binding, rafts could enhance secretion through the type III secretion apparatus (secreton) of effectors and favour signalling events required for Shigella uptake (Sansonetti, 2001).
Lipid rafts are indeed likely to do more than promote binding of Shigella to the host cell, as already suggested here by the observation that internalization is reduced after cholesterol extraction, in addition to impaired binding. A critical parameter for Shigella infection could be to reach a ‘signalling threshold’ below which any triggered signalling would be abortive and beyond which infection could proceed with further entry of the bacterium. Lipid rafts are ideal places for this to occur as they could favour recruitment of cytoskeleton regulators, in addition to their increasingly recognized role in signalling events (Simons and Toomre, 2000). It is therefore probable that the IpaB–CD44 complex not only allows Shigella–host binding but also initiates signalling cascades. Moreover, Shigella produces factors allowing the regulation of plasma membrane plasticity by activation/deactivation of signalling cascades (Dumenil et al., 2000). Raft partitioning of molecular complexes could offer an efficient way to interfere with the signalling network. It remains to be determined which part of this network is operating within rafts.
In conclusion, we demonstrate here that at least one protein complex involving a bacterial and a host protein is associated with lipid rafts and that these microdomains play an important role in early S.flexneri infection events. Further studies will be required to decipher which other molecular complexes are recruited within rafts and which cell signalling cascades are triggered during Shigella infection. Shigella flexneri therefore offers a model system of choice for the study of how raft dynamics participate in bacterial internalization.
Materials and methods
Cells and bacterial strains
HeLa and CHO.K1 cell lines were used as well as the SPB and SPB-1/cLCB1 cell lines described previously (Hanada et al., 1990). We used an E.coli K12 strain that expresses the invasin protein of Yersinia [E.coli (YInv+)] (Isberg and Leong, 1990) as well as the following S.flexneri strains: M90T, an invasive strain of S.flexneri serotype 5; BS176, a non-invasive derivative of M90T cured of the 220 kb virulence plasmid (Sansonetti et al., 1982); M90T-AfaE, corresponding to M90T transformed with the pILL1101 plasmid, which encodes an afimbrial adhesin of uropathogenic E.coli (Garcia et al., 1994); M90T-mxiD–, the M90T strain in which the mxiD gene has been inactivated (Tran Van Nhieu et al., 1997); M90T-GST and M90T-GST-IpaB, the SF 620 strain (Menard et al., 1993) transformed with pGEX2T (Pharmacia) alone or carrying the IpaB coding sequence (Chen et al., 1996), respectively. M90T-GFP is the M90T strain transformed with pEGFP (Clontech).
Antibodies and fluorescent probes
We used polyclonal and monoclonal anti-S.flexneri serotype 5 LPS antibodies, and polyclonal anti-IpaB (Mounier et al., 1997). Polyclonal anti-E.coli LPS antibodies and monoclonal anti-Rab5 antibody were kindly provided by C.Parsot (Pasteur Institut, Paris, France) and R.Jahn (MPI, Tübingen, Germany), respectively. Monoclonal anti-CD44 Hermes-1 epitope, which recognizes the hyaluronate binding site of CD44, and anti-CD44 Hermes-3 epitope, involved in lymphocyte binding to mucosal high endothelial venule (exon 5 CD44s standard form), were kindly provided by S.Jalkanen (University of Turku, Turku, Finland). Monoclonal anti-transferrin receptor antibody was from Zymed, polyclonal and monoclonal anti-caveolin-1, monoclonal anti-GST, polyclonal and monoclonal anti-Fyn and GAR–HRP antibodies were from Santa Cruz, GAR-Alexa488 from Molecular Probes, GAM–TRITC and SAR–TRITC from Jackson Immunoresearch, and SAM–HRP from Amersham. Hoechst dye was purchased from Molecular Probes, filipin and phalloidin–FITC from Sigma.
Fluorescence microscopy
Cells were fixed with 4% paraformaldehyde and when needed perme abilized with 0.1% Triton X-100 for 5 min at room temperature. Saturation medium was 10% phosphate-buffered saline–fetal calf serum (PBS–FCS). Cholesterol and GPI-anchored proteins were labelled respectively with filipin (15 µg/ml) and PA-Alexa546 conjugated (50 ng/ml) for 30 min at room temperature in 1% PBS–FCS. We used an inactive mutant of aerolysin, called ASSP, which retains GPI-binding activity (van der Goot et al., 1994). Coverslips, mounted in Mowiol (Sigma), were analysed with a Zeiss Axiophot fluorescence microscope using ×100 Plan-NEOFLUAR oil immersion objective. Pictures were acquired using the IPLab Spectrum software (Scientific Image Processing).
MeCD treatment
Cells were treated with 10 mM MeCD (Sigma) for 1 h at 37°C in incubation medium (IM: GMEM, HEPES 10 mM pH 7.4). Colorimetric quantification (kit from Roche) and TLC analysis (Abrami et al., 2001) showed that at least 70% of the total cholesterol was removed. Also, cells remained viable after MeCD treatment, as shown by Trypan Blue exclusion and Live/Dead assay (Molecular Probes).
Rec GST–IpaB production
The IpaB sequence inserted in pGEX2T was a generous gift from A.Zychlinsky (MPI, Berlin, Germany). Rec GST–IpaB was produced in S.flexneri SF620 strain upon induction with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 4 h. Rec GST–IpaB was purified after elution with reduced glutathione (Fluka) from a Sepharose-coupled GST (Pharmacia) column; the final concentration was routinely 0.5 µg/ml. Due to proteolysis, only 10–20% of the recombinant protein was full length (80 kDa) (the concentrations indicated refer to that of this high molecular weight form).
Recombinant protein binding and Shigella binding/entry quantitation
All incubations were carried out in IM. Recombinant proteins were used at between 20 and 80 nM giving similar results. For competition experiments, anti-CD44 (Hermes-1) antibody was used at 500 nM. For clustering experiments, anti-GST and TRITC-conjugated anti-mouse antibodies were used at 25 and 250 nM, respectively. Cell scraping was performed in TNE (Tris 10 mM pH 7.4, NaCl 150 mM, EDTA 5 mM) plus protease inhibitor (Complete, Roche). Infection was carried out according to established protocols for invasive and non-invasive strains (Clerc and Sansonetti, 1987). Briefly, overnight cultures of S.flexneri were diluted in trypticase–soy broth (Beckman Dickinson) at a 1:100 dilution and grown to mid-exponential phase in a shaking incubator. When M90T and BS176 S.flexneri strains were used, bacteria were resuspended in IM at a concentration of 2 × 108 bacteria/ml and added to the cells. Samples were centrifuged for 10 min at 800 g and incubated at 37°C for the times indicated. Shigella flexneri M90T-AfaE and M90T-mxiD– strains and E.coli (Yinv+) were resuspended at a concentration of 5 × 106 bacteria/ml and added to the cells. Entry efficiency was determined according to Skoudy et al. (2000), except that bound and internalized bacteria on one hand and only bound bacteria on the other hand were counted.
DRM preparation and immunoblotting analysis were performed as described in Lafont and Simons (2001). Briefly, membranes were solubilized with 0.5% Triton X-100 on ice for 30 min, a 40%, 30%, 0% Optiprep density step gradient was used to float membranes in Beckmann TLS 55 tubes for 2 h at 4°C at 55 000 r.p.m. No Triton X-100 was added in the OptiPrep gradient. Fractions were collected from the top and proteins were precipitated using a MetOH/CHCl3-MetOH procedure (Wessel and Flugge, 1984).
Acknowledgments
Acknowledgements
L.Abrami and J.Gruenberg are acknowledged for critical reading of the manuscript. This work was supported by a grant from the Swiss National Science Foundation to F.G.v.d.G.
References
- Abrami L., Fivaz,M., Kobayashi,T., Kinoshita,T., Parton,R.G. and van der Goot,F.G. (2001) Cross-talk between caveolae and glycosyl phosphatidylinositol-rich domains. J. Biol. Chem., 276, 30729–30736. [DOI] [PubMed] [Google Scholar]
- Adam T., Arpin,M., Prevost,M.C., Gounon,P. and Sansonetti,P.J. (1995) Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol., 129, 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H.A., Chen,Y. and Norkin,L.C. (1996) Bound simian virus 40 translocates to caveolin-enriched membrane domains and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell, 7, 1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baorto D.M., Gao,Z., Malaviya,R., Dustin,M.L., van der Merwe,A., Lublin,D.M. and Abraham,S.N. (1997) Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature, 389, 636–639. [DOI] [PubMed] [Google Scholar]
- Blocker A., Jouihri,N., Larquet,E., Gounon,P., Ebel,F., Parsot,C., Sansonetti,P. and Allaoui,A. (2001) Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol., 39, 652–663. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Catron D.M, Sylvester,M.D., Lange,Y., Kadekoppala,M., Jones,B.D., Monack,D.M., Falkow,S. and Haldar,K. (2002) The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell. Microbiol., 4, 315–328. [DOI] [PubMed] [Google Scholar]
- Chen Y., Smith,M.R., Thirumalai,K. and Zychlinkski,A. (1996) A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J., 15, 3853–3860. [PMC free article] [PubMed] [Google Scholar]
- Clerc P. and Sansonetti,P.J. (1987) Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect. Immun., 55, 2681–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenil G., Sansonetti,P. and Tran Van Nhieu,G. (2000) Src tyrosine kinase activity down-regulates Rho-dependent responses during Shigella entry into epithelial cells and stress fibre formation. J. Cell Sci., 113, 71–80. [DOI] [PubMed] [Google Scholar]
- Fivaz M., Abrami,L. and van der Goot,F.G. (1999) Pathogens, toxins and lipid rafts. Protoplasma, 212, 8–14. [DOI] [PubMed] [Google Scholar]
- Fivaz M., Vilbois,F., Thurnheer,S., Pasquali,C., Abrami,L., Bickel,P.E., Parton,R.G. and van der Goot,F.G. (2002) Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J., 21, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Labigne,A. and Le Bouguénec,C. (1994) Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J. Bacteriol., 176, 7601–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M.J., Hayward,R.D. and Koronakis,V. (2002) The Salmonella pathogenicity island 1 secretion system directs cellular cholesterol redistribution during mammalian cell entry and intracellular trafficking. Cell. Microbiol., 4, 153–165. [DOI] [PubMed] [Google Scholar]
- Gatfield J. and Pieters,J. (2000) Essential role for cholesterol in entry of mycobacteria into macrophages. Science, 288, 1647–1650. [DOI] [PubMed] [Google Scholar]
- Guignot J., Peiffer,I., Bernet-Camard,M.F., Lublin,D.M., Carnoy,C., Moseley,S.L. and Servin,A.L. (2000) Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun., 68, 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignot J., Bernet-Camard,M.F., Pous,C., Plancon,L., Le Bouguenec,C. and Servin,A.L. (2001) Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for α5β1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun., 69, 1856–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Nishijima,M. and Akamatsu,Y. (1990) A temperature-sensitive mammalian cell mutant with thermolabile serine palmitoyltransferase for the sphingolipid biosynthesis. J. Biol. Chem., 265, 22137–22142. [PubMed] [Google Scholar]
- Hanada K., Hara,T., Nishijima,M., Kuge,O., Dickson,R.C. and Nagiec,M.M. (1997) A mammalian homolog of the yeast LCB1 encodes a component of serine palmitoyltransferase, the enzyme catalyzing the first step in sphingolipid synthesis. J. Biol. Chem., 272, 32108–32114. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele,P., Verkade,P. and Simons,K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M., Sato,N., Kondo,T., Yonemura,S., Monden,M., Sasaki,T., Takai,Y. and Tsukita,S. (1996) Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol., 135, 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R.R. and Leong,J.M. (1990) Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell, 60, 861–871. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok,H. and Helenius,A. (1989) Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol., 109, 2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F. and Simons,K. (2001) Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl Acad. Sci. USA, 98, 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R., Sansonetti,P.J. and Parsot,C. (1993) Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol., 175, 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Bahrani,F. and Sansonetti,P.J. (1997) Secretion of Shigella flexneri Ipa invasins on contact with epithelial cells and subsequent entry of the bacterium into cells are growth stage dependent. Infect. Immun., 65, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Laurent,V., Hall,A., Fort,P., Carlier,M.F., Sansonetti,P.J. and Egile,C. (1999) Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci., 112, 2069–2080. [DOI] [PubMed] [Google Scholar]
- Neame S.J. and Isacke,C.M. (1993) The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J. Cell Biol., 121, 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L.C., Wolfrom,S.A. and Stuart,E.S. (2001) Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res., 266, 229–238. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Hart,A., Coyne,K.E., Lublin,D.M. and Nowicki,S. (1993) Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell–cell interaction. J. Exp. Med., 178, 2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S., Paiha,K., Harder,T., Gerke,V., Schwarzler,C., Schwarz,H., Beug,H., Gunthert,U. and Huber,L.A. (1999) Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J. Cell Biol., 146, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C., Menard,R., Gounon,P. and Sansonetti,P.J. (1995) Enhanced secretion through the Shigella flexneri Mxi–Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol. Microbiol., 16, 291–300. [DOI] [PubMed] [Google Scholar]
- Rosenberger C.M., Brumell,J.H. and Finlay,B.B. (2000) Microbial pathogenesis: lipid rafts as pathogen portals. Curr. Biol., 10, R823–R825. [DOI] [PubMed] [Google Scholar]
- Sansonetti P.J. (2001) Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote– eukaryote cross-talks. FEMS Microbiol. Rev., 25, 3–14. [DOI] [PubMed] [Google Scholar]
- Sansonetti P.J., Kopecko,D.J. and Formal,S.B. (1982) Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun., 35, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P.J., Ryter,A., Clerc,P., Maurelli,A.T. and Mounier,J. (1986) Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun., 51, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons K. and Toomre,D. (2000) Lipid rafts and signal transduction. Nature Rev. Mol. Cell Biol., 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Skoudy A., Nhieu,G.T., Mantis,N., Arpin,M., Mounier,J., Gounon,P. and Sansonetti,P. (1999) A functional role for ezrin during Shigella flexneri entry into epithelial cells. J. Cell Sci., 112, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Skoudy A., Mounier,J., Aruffo,A., Ohayon,H., Gounon,P., Sansonetti,P. and Tran Van Nhieu,G. (2000) CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell. Microbiol., 2, 19–33. [DOI] [PubMed] [Google Scholar]
- Stang E., Kartenbeck,J. and Parton,R.G. (1997) Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell, 8, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. and Sasakawa,C. (2001) Molecular basis of the intracellular spreading of Shigella. Infect. Immun., 69, 5959–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Ben-Ze’ev,A. and Sansonetti,P.J. (1997) Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J., 16, 2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Goot F.G., Hardie,K.R., Parker,M.W. and Buckley,J.T. (1994) The C-terminal peptide produced upon proteolytic activation of the cytolytic toxin aerolysin is not involved in channel formation. J. Biol. Chem., 269, 30496–30501. [PubMed] [Google Scholar]
- Wary K.K., Mariotti,A., Zurzolo,C. and Giancotti,F.G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell, 94, 625–634. [DOI] [PubMed] [Google Scholar]
- Watarai M., Funato,S. and Sasakawa,C. (1996) Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med., 183, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M., Kamata,Y., Kozaki,S. and Sasakawa,C. (1997) rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J. Exp. Med., 185, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D. and Flugge,U.I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem., 138, 141–143. [DOI] [PubMed] [Google Scholar]