Abstract
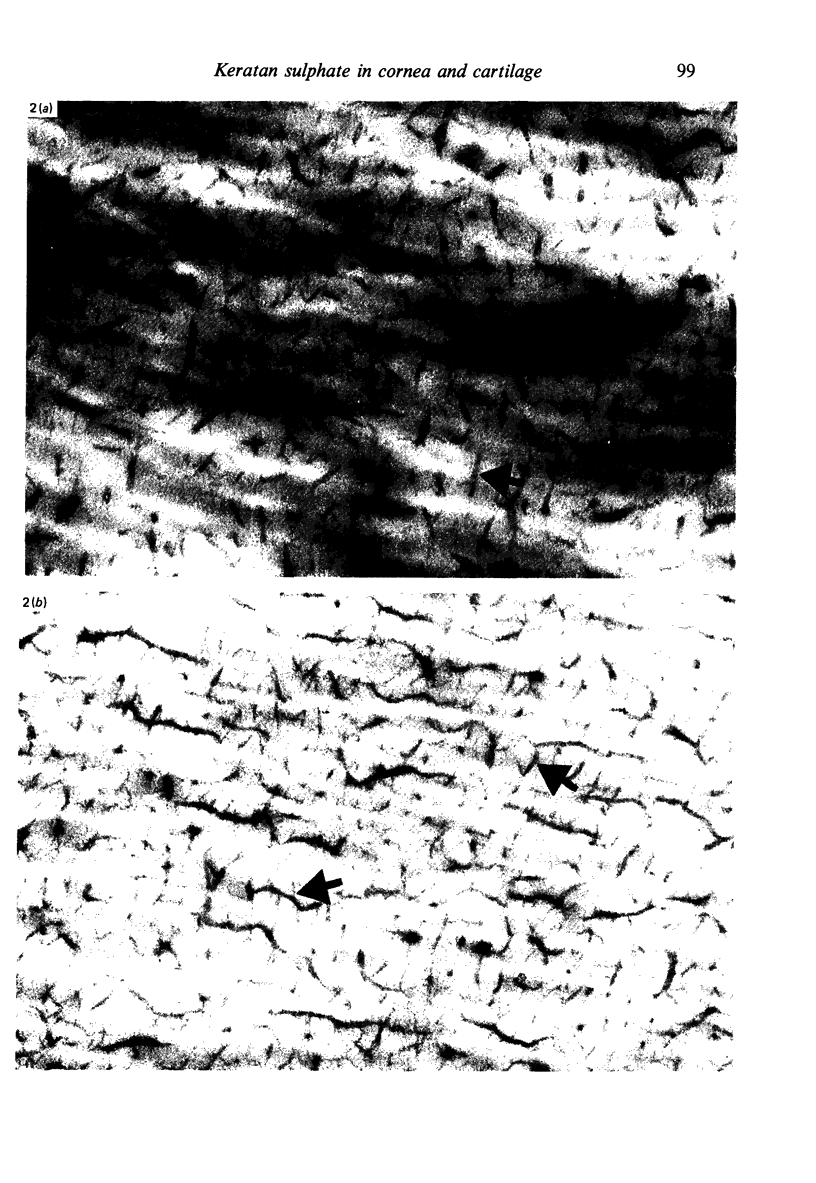
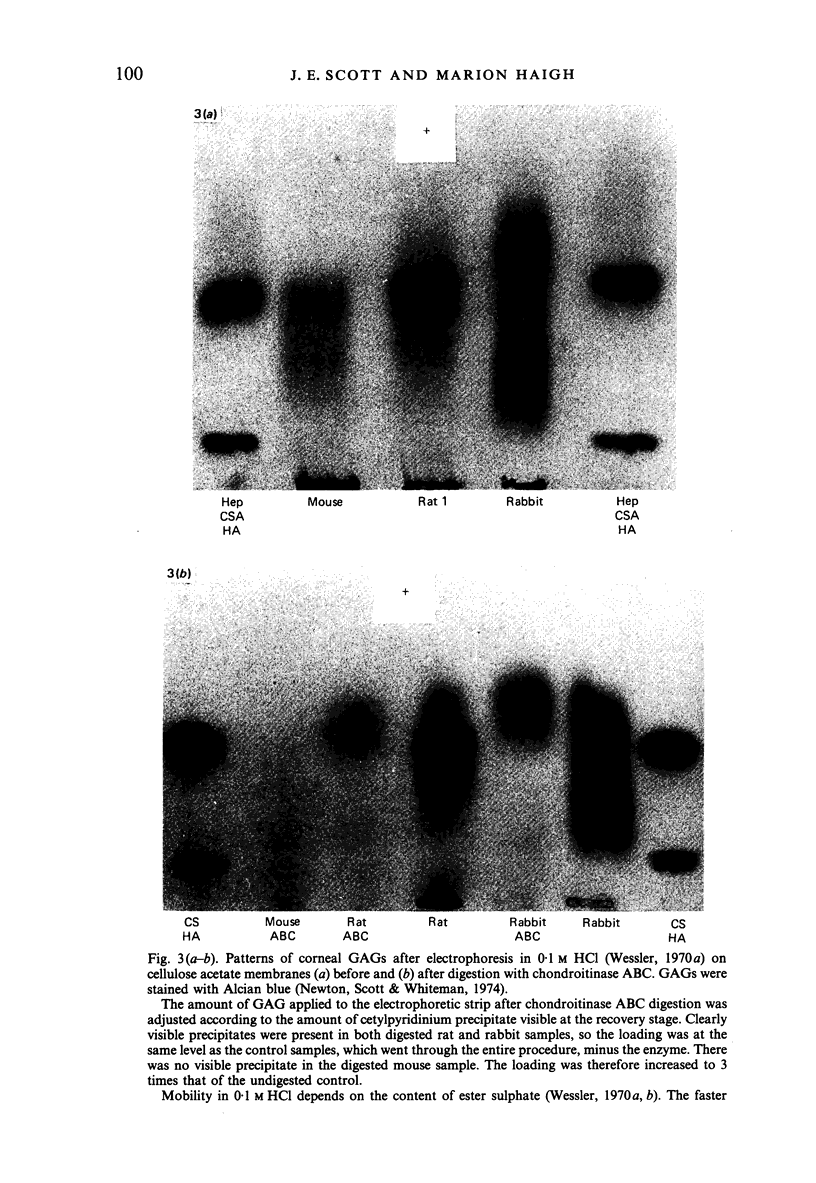
Corneas from mouse, rat and rabbit were analysed quantitatively and/or qualitatively for collagen and acid glycosaminoglycans. They were examined by light and electron microscopy, using Alcian blue and Cupromeronic blue, in critical electrolyte concentration methods, with or without digestion by hyaluronidase, chondroitinases and keratanase, for their sulphated glycosaminoglycan distributions. Glycosaminoglycan patterns were very different in the three species. Mouse lacked chemically detectable keratan sulphate, which was present in considerable amounts in rat and rabbit stroma. Mouse corneal stroma proteoglycan filaments were located predominantly at the gap zone of the collagen fibrils, mainly at the d band, with few at the a and c bands. Rat and rabbit micrographs were more complicated, with many proteoglycan filaments at the a and c, as well as the d and e bands. These findings support the proposal that the a and c bands were specific binding sites for keratan sulphate proteoglycan (Scott & Haigh, 1985b). Evidence from studies on cornea and cartilage suggests that keratan sulphate, rather than chondroitin sulphate is produced in conditions of O2 lack. Metabolic mechanisms which could account for this balance are proposed The production of uridine diphosphate glucuronic acid is the key step, which is sensitive to hypoxia, lactate and NAD:NADH ratios.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSETH A., LAURENT T. C. Studies on corneal polysaccharides. I. Separation. Exp Eye Res. 1961 Sep;1:25–38. doi: 10.1016/s0014-4835(61)80005-5. [DOI] [PubMed] [Google Scholar]
- ANSETH A. Studies on corneal polysaccharides. III. Topographic and comparative biochemistry. Exp Eye Res. 1961 Dec;1:106–115. doi: 10.1016/s0014-4835(61)80015-8. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Jones D. P. Control of glucuronidation during hypoxia. Limitation by UDP-glucose pyrophosphorylase. Biochem J. 1984 May 1;219(3):707–712. doi: 10.1042/bj2190707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balduini C., Brovelli A., Cstellani A. A. Biosynthesis of glycosaminoglycans in bovine cornea. The effet of uridine diphosphate xylose. Biochem J. 1970 Dec;120(4):719–723. doi: 10.1042/bj1200719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini C., Brovelli A., De Luca G., Galligani L., Castellani A. A. Uridine diphosphate glucose dehydrogenase from cornea and epiphysial-plate cartilage. Biochem J. 1973 Jun;133(2):243–249. doi: 10.1042/bj1330243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim F. A., Goetz D. Distribution of hexosamines in bovine cornea. Invest Ophthalmol. 1976 Apr;15(4):301–304. [PubMed] [Google Scholar]
- Borcherding M. S., Blacik L. J., Sittig R. A., Bizzell J. W., Breen M., Weinstein H. G. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res. 1975 Jul;21(1):59–70. doi: 10.1016/0014-4835(75)90057-3. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Distler J. J., Jourdian G. W. Biosynthesis of keratan sulfate: purification and properties of a galactosyltransferase from bovine cornea. Arch Biochem Biophys. 1979 Feb;192(2):548–558. doi: 10.1016/0003-9861(79)90125-5. [DOI] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl I. M., Cöster L. Proteoglycan biosynthesis in cultures of corneas and corneal stroma cells from adult rabbits. Exp Eye Res. 1978 Aug;27(2):175–190. doi: 10.1016/0014-4835(78)90087-8. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Anseth A. Studies on corneal polysaccharides. IV. Chromatography of corneal glycosaminoglycans on ECTEOLA cellulose using formate buffers as eluting solvents. Exp Eye Res. 1967 Apr;6(2):107–119. doi: 10.1016/s0014-4835(67)80061-7. [DOI] [PubMed] [Google Scholar]
- Gregory J. D., Cöster L., Damle S. P. Proteoglycans of rabbit corneal stroma. Isolation and partial characterization. J Biol Chem. 1982 Jun 25;257(12):6965–6970. [PubMed] [Google Scholar]
- HEDBLOM E. E. The role of polysaccharides in corneal swelling. Exp Eye Res. 1961 Sep;1:81–91. doi: 10.1016/s0014-4835(61)80012-2. [DOI] [PubMed] [Google Scholar]
- Haigh M., Scott J. E. A method of processing tissue sections for staining with cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30(4):479–486. [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Phelps C. F. The biosynthesis in vitro of keratan sulphate in bovine cornea. Biochem J. 1972 Jun;128(2):205–213. doi: 10.1042/bj1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Inerot S., Heinegård D. Bovine tracheal cartilage proteoglycans. Variations in structure and composition with age. Coll Relat Res. 1983 May;3(3):245–262. doi: 10.1016/s0174-173x(83)80007-7. [DOI] [PubMed] [Google Scholar]
- KAPLAN D., MEYER K. Ageing of human cartilage. Nature. 1959 May 2;183(4670):1267–1268. doi: 10.1038/1831267a0. [DOI] [PubMed] [Google Scholar]
- Keller R., Stein T., Stuhlsatz H. W., Greiling H., Ohst E., Müller E., Scharf H. D. Studies on the characterization of the linkage-region between polysaccharide chain and core protein in bovine corneal proteokeratan sulfate. Hoppe Seylers Z Physiol Chem. 1981 Mar;362(3):327–336. doi: 10.1515/bchm2.1981.362.1.327. [DOI] [PubMed] [Google Scholar]
- MAURICE D. M. The structure and transparency of the cornea. J Physiol. 1957 Apr 30;136(2):263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER K., LINKER A., DAVIDSON E. A., WEISSMANN B. The mucopolysaccharides of bovine cornea. J Biol Chem. 1953 Dec;205(2):611–616. [PubMed] [Google Scholar]
- Meek K. M., Elliott G. F., Nave C. A synchrotron X-ray diffraction study of bovine cornea stained with cupromeronic blue. Coll Relat Res. 1986 Jun;6(2):203–218. doi: 10.1016/s0174-173x(86)80026-7. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Andersson B., Norling A. Interaction of ethanol oxidation with glucuronidation in isolated hepatocytes. Biochem Pharmacol. 1978;27(22):2583–2588. doi: 10.1016/0006-2952(78)90331-3. [DOI] [PubMed] [Google Scholar]
- Newton D. J., Scott J. E., Whiteman P. The estimation of acid glycosaminoglycan-Alcian blue complexes eluted from electrophoretic strips. Anal Biochem. 1974 Nov;62(1):268–273. doi: 10.1016/0003-2697(74)90386-8. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-collagen interactions in intervertebral disc. A chondroitin sulphate proteoglycan associates with collagen fibrils in rabbit annulus fibrosus at the d-e bands. Biosci Rep. 1986 Oct;6(10):879–888. doi: 10.1007/BF01116241. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I. A. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A. Changes in the acid glycosaminoglycan content of the matrix of ageing human articular cartilage. Ann Rheum Dis. 1970 Sep;29(5):509–515. doi: 10.1136/ard.29.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Distribution of acid glycosaminoglycans in human articular cartilage. Nature. 1967 Sep 23;215(5108):1376–1378. doi: 10.1038/2151376a0. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlsatz H. W., Hirtzel F., Keller R., Cosma S., Greiling H. Studies on the polydispersity and heterogeneity of proteokeratan sulfate from calf and porcine cornea. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):841–852. doi: 10.1515/bchm2.1981.362.2.841. [DOI] [PubMed] [Google Scholar]
- Venn G., Mason R. M. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 1985 Jun 1;228(2):443–450. doi: 10.1042/bj2280443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Ward N. P., Scott J. E., Cöster L. Dermatan sulphate proteoglycans from sclera examined by rotary shadowing and electron microscopy. Biochem J. 1987 Mar 15;242(3):761–766. doi: 10.1042/bj2420761. [DOI] [PMC free article] [PubMed] [Google Scholar]