Abstract
We examined the roles of inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) in calcium signaling using DT40 B lymphocytes, and a variant lacking the three IP3R isoforms (IP3R-KO). In wild-type cells, B cell receptor (BCR) stimulation activates a cation entry route that exhibits significantly greater permeability to Ba2+ than does capacitative calcium entry. This cation entry is absent in IP3R-KO cells. Expression of the type-3 IP3R (IP3R-3) in the IP3R-KO cells rescued not only agonist-dependent release of intracellular Ca2+, but also Ba2+ influx following receptor stimulation. Similar results were obtained with an IP3R-3 mutant carrying a conservative point mutation in the selectivity filter region of the channel (D2477E); however, an IP3R-3 mutant in which this same aspartate was replaced by alanine (D2477A) failed to restore either BCR-induced Ca2+ release or receptor-dependent Ba2+ entry. These results suggest that in DT40 B lymphocytes, BCR stimulation activates a novel cation entry across the plasma membrane that depends upon, or is mediated by, fully functional IP3R.
Keywords: B lymphocyte/calcium channels/inositol trisphosphate receptor/phospholipase C/plasma membrane
Introduction
Activation of the phospholipase C pathway in non-excitable cells results in the generation of inositol 1,4,5-trisphosphate (IP3), which stimulates the release of Ca2+ store in the endoplasmic reticulum (Berridge, 1993). This release of Ca2+ is generally associated with an increase in Ca2+ entry across the plasma membrane that can serve to replenish stores or to contribute to Ca2+-dependent signaling. In the majority of instances, this entry of Ca2+ appears to be signaled through a poorly understood mechanism that is initiated by depletion of the Ca2+ stores (Putney, 1986), a process known as capacitative calcium entry (CCE) or store-operated calcium entry (Putney, 1997a).
Non-CCE mechanisms have been described in a variety of cell types (Fasolato et al., 1993; Byron and Taylor, 1995; Shuttleworth and Thompson, 1996; Carroll and Peralta, 1998; Broad et al., 1999). The mechanisms controlling Ca2+ channel activation in such instances are not known, but may involve other second messengers, for example arachidonic acid. One second messenger that has been considered as a signal for activating plasma membrane Ca2+ entry is IP3 acting on plasma membrane IP3 receptors (IP3R). In fact, a number of studies have provided biochemical evidence for the presence of IP3R in the plasma membrane of cells (Guillemette et al., 1988; Fujimoto et al., 1992; Khan et al., 1992a,b, 1996; Feng and Kraus-Friedmann, 1993; Bush et al., 1994; Mayrleitner et al., 1995; Quinton and Dean, 1996; El-Daher et al., 2000; Tanimura et al., 2000). However, there is little physiological evidence that these receptors play a significant role in Ca2+ signaling at the plasma membrane. The one notable exception is vertebrate and invertebrate olfactory neurons, for which there is considerable functional evidence for the presence of plasma membrane IP3-gated channels (Cunningham et al., 1993; Fadool and Ache, 1994; Okada et al., 1994; Kashiwayanagi, 1996; Lischka et al., 1999; Munger et al., 2000). A role for the IP3R type-3 (IP3R-3) has been proposed as responsible for capacitative Ca2+ entry in some cell types (Putney, 1997b), but it is clear this cannot be the case in all cell types (Wang et al., 2001).
Both T and B lymphocytes have been shown, by immunostaining, to possess plasma membrane IP3R, and these receptors have been suggested to play a role in apoptosis (Khan et al., 1992a,b, 1996). We have investigated the role of IP3R in plasma membrane calcium fluxes, utilizing the DT40 B lymphocyte cell line. This is an excellent model for investigating the function of IP3R in B cell signaling because of the availability of a mutant DT40 line lacking all three IP3R isoforms (Sugawara et al., 1997). DT40 is a chicken B cell line expressing an IgM isotype B cell receptor (BCR) in the plasma membrane (Baba et al., 1985) that is linked to intracellular Ca2+ ([Ca2+]i) signaling through phospholipase Cγ (PLCγ) activation (Buerstedde and Takeda, 1991; Sugawara et al., 1997). PLCγ activation results in conversion of phosphatidylinositol 4,5-bisphosphate into IP3, which then releases Ca2+ from thapsigargin-sensitive intracellular stores by interacting with specific IP3R in the endoplasmic reticulum (Berridge, 1993). This release results in transient elevation of cytosolic calcium concentration and is followed by a more sustained Ca2+ entry from the outside, which is mediated, at least in part, by the store-operated, CCE pathway (Putney, 1986; Berridge, 1995; Parekh and Penner, 1997; Barritt, 1999; Sugawara et al., 1997). In the present work we demonstrate that there is also a non-capacitative pathway for calcium entry in DT40 B lymphocytes, activated through the BCR. This entry pathway, while not mediated by store depletion, requires a functional IP3R. In light of previous work providing biochemical evidence for IP3R in the plasma membrane of B cells and other cell types, we suggest that this entry of divalent cations may result from direct gating of plasma membrane IP3R by IP3.
Results
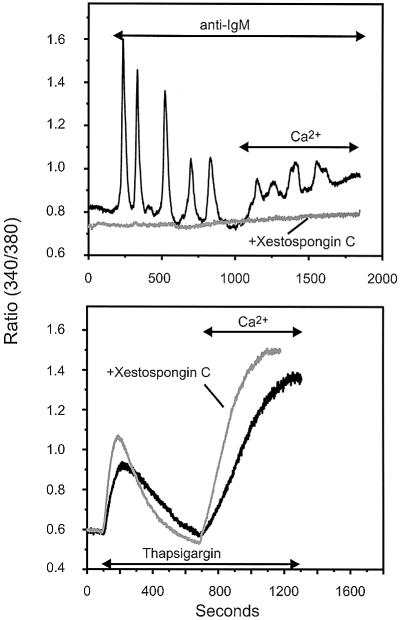
As shown in Figure 1, activation of the BCR in DT40 B lymphocytes, in the absence of extracellular Ca2+, results in an oscillatory [Ca2+]i signal, presumably due to cyclical release of Ca2+ from intracellular stores. In the current study, Ca2+ responses to BCR activation were observed in 60–70% of cells tested (n = 195). Restoration of extracellular Ca2+ to the responsive cells revealed a stimulated entry of Ca2+ in 80% of the BCR-responsive cells. Both the release and entry of Ca2+ appear to depend on IP3R in some manner, because both phases of the response are lost in cells lacking IP3R (Sugawara et al., 1997), and both phases are completely blocked by the potent membrane permeant IP3R inhibitor, xestospongin C (Gafni et al., 1997; Figure 1, top). Release of Ca2+ by the SERCA inhibitor, thapsigargin, and the resulting CCE, were not inhibited by xestospongin C (Figure 1, bottom).
Fig. 1. The IP3R inhibitor xestospongin C effectively suppresses both BCR-induced Ca2+ release and calcium entry in DT40 cells B lymphocytes, but does not affect thapsigargin-induced Ca2+ release or entry. DT40 cells were incubated for 45 min in the presence of 25 µM xestospongin C. Cells were then bathed in nominally Ca2+-free medium, exposed to 5 µg/ml anti-IgM antibody (top) or 2 µM thapsigargin (bottom), as indicated. Ca2+ (1.5 mM) was added where indicated. In this experiment, relatively higher ratio values were obtained as compared with other experiments due to the use of a different Ca2+ measuring system; in this series (and for experiments in Figure 3), a photon counting system was used, while in the other series, an imaging system was used. Black trace: control (no xestospongin C); gray trace, 25 µM xestospongin C. Shown are representative traces from three independent experiments.
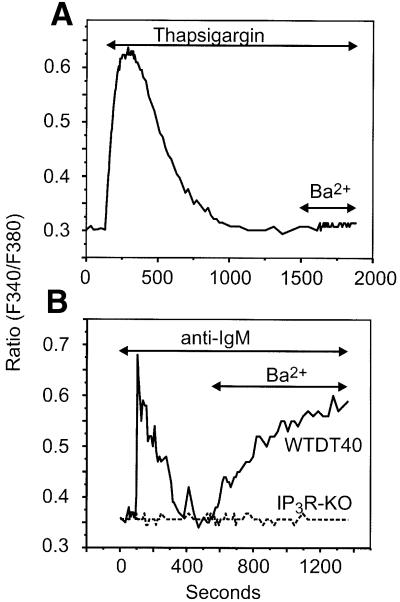
The function of IP3R in the Ca2+ entry phase could be due to their role in the release of store Ca2+ and activation of store-operated channels, or alternatively there may be some other, distinct mechanism for regulation of Ca2+ by IP3R. If the latter were the case, then one prediction is that BCR stimulation should activate an entry in addi tion to that, dependent solely on Ca2+ store depletion. Furthermore, this response should be absolutely dependent on the presence of IP3R. This appears to be the case, as shown by results in Figure 2. In these experiments, we took advantage of the fact that under our experimental conditions, the store-operated Ca2+ entry pathway involves either no detectable Ba2+ entry or, in some experiments, a small Ba2+ entry (Vazquez et al., 2001; data not shown; Figure 2A). However, unlike thapsigargin-activated CCE, BCR stimulation results in a substantial entry of Ba2+ entry (Figure 2B). This clearly indicates that BCR-dependent cation entry involves not only store-operated channels, but also a population of somewhat less selective cation channels requiring signals other than, or in addition to, store depletion.
Fig. 2. BCR activation, but not store depletion, induces Ba2+ entry in DT40 B lymphocytes. (A) Fura-2-loaded WTDT40 cells were incubated in nominally Ca2+-free medium and then exposed to 2 µM thapsigargin in order to deplete intracellular Ca2+ stores. After cytosolic Ca2+ returned to basal levels, Ba2+ (10 mM) was added to the medium. A representative trace from at least five independent experiments is shown. (B) Wild-type (WTDT40) or IP3R-KO (broken line) DT40 cells were maintained in nominally Ca2+-free medium, exposed to 5 µg/ml anti-IgM antibody, and then Ba2+ (10 mM) was added where indicated. Representative traces from at least five independent experiments are shown.
In experiments utilizing Ba2+ to assess entry, 102 of 127 anti-IgM responsive cells showed Ba2+ entry. Note that in a previous publication, Venkatachalam et al. (2001) failed to observe agonist-activated Ba2+ entry. This difference is most likely due to the fact that Venkatachalam and colleagues used 1 mM Ba2+, while in our experiments the concentration of Ba2+ was 10 mM.
The failure to observe cation entry in all anti-IgM-responsive cells could result from rapid desensitization of the receptor–phospholipase C pathway. In fact, desensitization of the B cell antigen receptor may occur with as few as 5% of the receptors occupied, resulting in blockade of the signaling pathways derived from receptor activation by a mechanism apparently involving a short-term action of PKC (Vilen et al., 1997). There was no detectable Ba2+ entry in those cells unable to respond to BCR crosslinking (68 of 68 cells). Importantly, the receptor-dependent Ba2+ entry was never observed in IP3R knockout cells (IP3R-KO, a variant lacking the three IP3R isoforms, IP3R-1, -2 and -3) (Figure 2B), indicating that the receptor-activated cation influx is dependent on the presence of IP3R rather than depletion of Ca2+ stores.
In the remaining experiments, we took advantage of the apparent higher Ca2+ selectivity of the endogenous store-operated channels in order to assess BCR-dependent cation (Ba2+) entry into the cells without contribution from receptor activated endogenous CCE channels (see for example Vazquez et al., 2001). In addition, as Ba2+ is a poor substrate for endoplasmic reticulum or plasma membrane calcium pumps (Vanderkooi and Martonosi, 1971; Yamaguchi et al., 1989) and thus is not readily cleared from the cytoplasm, it provides a reliable way to monitor unidirectional cation entry that avoids potential complications derived from changes in cellular Ca2+ metabolism or Ca2+-dependent regulation of the cation channels (Byron and Taylor, 1995).
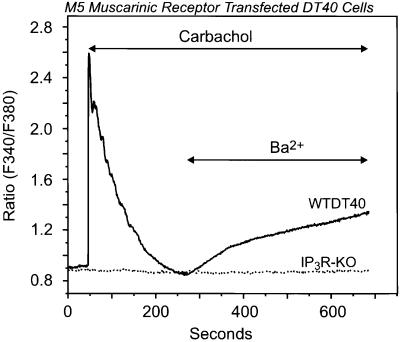
The failure of BCR stimulation to activate entry in IP3R-KO cells indicates the importance of the IP3R in this response, but does not rule out contributions of other elements of the BCR signaling pathway. Thus, we next transiently transfected DT40 cells with the human M5 muscarinic receptor, which would activate a different PLC, PLCβ, through a G-protein rather than tyrosine kinase-dependent mechanism. As shown in Figure 3, carbachol stimulation of the M5-expressing cells resulted in a rapid and transient cytosolic calcium rise (12 of 17 EYFP+ cells), which rapidly declined to basal levels in ∼2 min; addition of Ba2+ to the external medium resulted in significant cation entry, which was not observed in those cells unable to respond to muscarinic stimulation. Again, this receptor-dependent Ba2+ entry was never observed in IP3R-KO cells transfected with the M5 receptor. These results indicate that the cation entry route following receptor activation was related to IP3 production and IP3R expression, but not linked to the type of receptor eliciting the signal.
Fig. 3. Activation of the G-protein-coupled M5 muscarinic receptor results in stimulation of Ba2+ entry in wild-type but not in IP3R-KO DT40 cells. Ba2+ influx was measured in single Fura-2-loaded wild-type (WTDT40) or IP3R-KO (dotted line) DT40 cells transfected with the human M5 muscarinic receptor. The cells were maintained in nominally Ca2+-free medium, exposed to 100 µM carbachol, and then Ba2+ (10 mM) was added where indicated. The relatively higher ratio values in this experiment are due to the use of a different Ca2+ measuring system; in this series, a photon counting system was used, while in the other series, an imaging system was used. Representative traces from three independent experiments are shown.
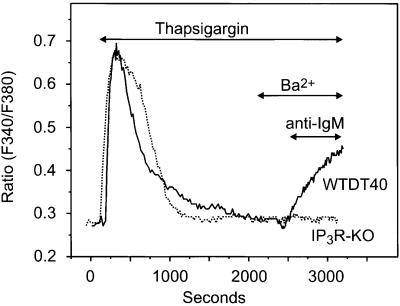
We next considered the possibility that activation of Ba2+ entry following BCR crosslinking could be the consequence of activation of Ca2+-activated non-selective cation channels due to the transient cytosolic Ca2+ rise that follows IP3-mediated Ca2+ mobilization from inner stores. In order to demonstrate clearly the dependence of the Ba2+ entry on BCR stimulation, we utilized the following protocol. Both wild-type (WTDT40) and IP3R-KO cells were treated with thapsigargin to discharge intracellular stores completely. Ba2+ was then added and, as shown previously, no detectable entry of Ba2+ occurred. Subsequently, anti-IgM was added, and in the WTDT40, but not in the IP3R-KO cells, Ba2+ entry occurred almost immediately (Figure 4). Interestingly, with this protocol a greater proportion of the cells (67 of 71) exhibited significant cation entry upon BCR stimulation, probably due to the fact that Ba2+ entry is triggered almost as soon as the receptor pathway becomes activated, thus minimizing any possible desensitization of the receptor signaling route.
Fig. 4. BCR-dependent activation of Ba2+ entry in wild-type but not in IP3R-KO DT40 cells. Fura-2-loaded wild-type (WTDT40) and IP3R-KO DT40 cells were incubated in nominally Ca2+-free medium and then exposed to 2 µM thapsigargin. After complete store depletion, Ba2+ (10 mM) was added to the medium where indicated, and 2 min later anti-IgM (5 µg/ml) was added. Representative traces from three independent experiments are shown.
One interesting point that can be taken from the protocol shown in Figure 4 is that the delay in Ba2+ entry is noticeably briefer than that for release following BCR activation. The average delay for the increase in Ba2+ entry after anti-IgM addition from the experiments in Figure 4 was 18 ± 10 s, while the delay for release from various protocols varied from 40 to 60 s. This is perhaps not surprising, if the entry is due to IP3 formed and acting at the plasma membrane, while release results from an action of IP3 at the endoplasmic reticulum.
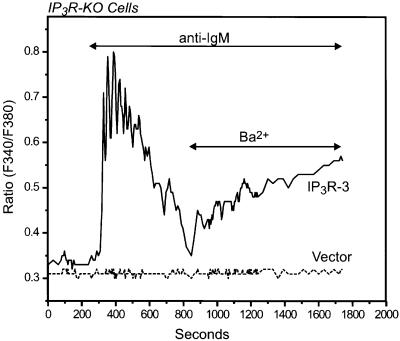
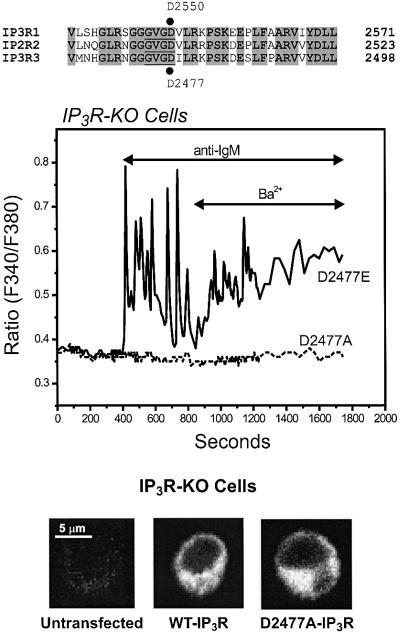
To confirm that it is the IP3R that is necessary for the BCR-dependent Ba2+ entry in DT40 cells, we attempted to restore this entry mechanism in IP3R-KO cells by transient expression of the rat IP3R-3. As predicted, stimulation of BCR signaling in IP3R-3-expressing IP3R-KO cells resulted not only in reappearance of the BCR-dependent, IP3-mediated release of stored Ca2+, but also in a significant Ba2+ entry following receptor activation (21 of 26 IgM-responsive EYFP+ cells; Figure 5). Similarly, carbachol stimulation of IP3R-KO cells transiently co-transfected with the M5 muscarinic receptor and the rat IP3R-3 resulted in rapid release of calcium from stores followed by activation of the Ba2+ entry route in response to carbachol (data not shown). Neither the transient Ca2+ release nor the Ba2+ entry was detected in mock-transfected IP3R-KO cells. These results indicate that in DT40 B lymphocytes, activation of the BCR signaling pathway results in generation of IP3, which in turn activates not only the endoplasmic reticulum-located IP3R that cause Ca2+ release from endogenous stores, but also a cation entry pathway in the plasma membrane strongly linked to IP3R expression. Unlike the highly selective CRAC channels, the IP3R is a relatively less selective cation channel more permeable to Ba2+ than to Ca2+ (Bezprozvanny and Ehrlich, 1994, 1995). Thus, the simplest explanation for this IP3R-dependent entry is that it represents cation flux through plasma membrane IP3R. Although this would appear to be the most straightforward explanation for the current observations, we cannot rule out an indirect regulation of a plasma membrane channel by IP3R, as in the conformational coupling mechanism for TRP or store-operated channels (Irvine, 1990; Berridge, 1995; Kiselyov et al., 1998). In an attempt to resolve this issue we transiently expressed two different pore mutants of the IP3R-3 into IP3R-KO cells and tested their abilities to restore BCR stimulation of the Ba2+ entry pathway. It has recently been shown that the aspartic acid residue at position 2550 of the rat IP3R-1 plays a critical role in cation permeation through the channel (Boehning and Joseph, 2000). This amino acid is located within a highly conserved sequence in the IP3R (GVGD) that is homologous to the selectivity filter in potassium channels (Doyle et al., 1998; see diagram in Figure 6). Replacing this aspartate by alanine (D2550A) results in an IP3R that is still able to bind IP3 but devoid of Ca2+ release activity, i.e. rendering an impermeable channel. A conservative mutation in which the aspartate is replaced by a glutamic acid (D2550E) fully preserves the IP3-induced Ca2+ release activity of the channel. These mutations do not alter the ability of the channels to express at high levels or to form oligomers (Boehning and Joseph, 2000). Unfortunately, we have not as yet successfully expressed the rat IP3R-1 in DT40 IP3R-KO cells. Thus, we engineered these same two point mutations in the equivalent aspartate residue at position 2477 in the rat IP3R-3 (mutants D2477A and D2477E, respectively). As shown in Figure 6, the D2477A mutant, when expressed in IP3R-KO cells, failed to restore either BCR-induced Ca2+ release or receptor-dependent Ba2+ entry. Heterologous expression of the D2477A IP3R-3 mutant was confirmed by immunocytochemistry (Figure 6), and the extent of expression and localization of the mutant appeared to be identical to that of the wild-type IP3R-3. Unfortunately, because of the small size of DT40 and the expression of IP3R-3 on endoplasmic reticulum through the non-nuclear regions, we were not able to definitively localize the receptor to the plasma membrane by immunocytochemistry.
Fig. 5. Transient expression of an IP3R restores agonist-induced Ba2+ entry in IP3R-KO DT40 cells. Ba2+ influx was measured in Fura-2-loaded IP3R-KO DT40 cells transfected with either the rat IP3R-3 or its vector (Mock). The cells were maintained in nominally Ca2+-free medium, exposed to 5 µg/ml anti-IgM antibody, and then Ba2+ (10 mM) was added where indicated. Representative traces from three independent experiments are shown.
Fig. 6. Effect of single point mutations within the putative pore forming region of IP3R-3 on restoration of agonist-induced Ba2+ entry in IP3R-KO DT40 cells. The top of the figure shows an alignment of a region including the predicted pore of rat IP3R-1–3 (the putative selectivity filter sequence is underlined). D2477 of rat IP3R-3, which was mutated into glutamate (D2477E) or alanine (D2477A), corresponds to D2550 of rat IP3R-1. In the experiment shown below, Ba2+ influx was measured in Fura-2-loaded IP3R-KO DT40 cells transfected with either the D2477E (solid line) or the D2477A (broken line) mutants of the rat IP3R-3 (see Materials and methods for details on the mutations). The cells were maintained in nominally Ca2+-free medium, exposed to 5 µg/ml anti-IgM antibody, and then Ba2+ (10 mM) was added where indicated. Representative traces from three independent experiments are shown. At the bottom, immunolocalization of transiently expressed rat IP3R-3 (pCB6+ vector) in DT40 B cells is shown. IP3R-KO cells untransfected, or transfected with the wild-type rat IP3R-3 (WT-IP3R), or the rat IP3R-3 mutant D2477A (D2477A-IP3R; see text for details) were incubated with a mouse anti-IP3R-3 monoclonal antibody and then with the secondary Alexa Fluor 488-labeled anti-mouse IgG antibody. The fluorescence images were acquired with a Zeiss 410 confocal microscope.
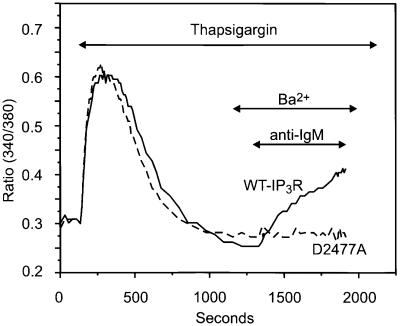
Because we cannot be certain that depletion of stores is not required for activation of this pathway, we also tested the D2477A mutant in a protocol in which Ca2+ stores were first depleted by thapsigargin; again, no BCR stimulation of Ba2+ entry was observed (Figure 7). The inability of this mutant to restore receptor-stimulated, IP3-mediated release of Ca2+ from intracellular stores is consistent with the expectation that replacement of the D2477 within the predicted selectivity region of the channel by a non-negatively charged amino acid would abrogate cation permeation through the channel pore. However, the D2477E mutant, in which the negative charge is preserved by replacing aspartate with glutamate, fully retained the phenotype exhibited by IP3R-KO cells transiently expressing the wild-type IP3R-3. Mutant or truncated IP3R lacking functional pores are known to be fully capable of activating plasma membrane channels through the conformational coupling mechanism (Kiselyov et al., 1998, 1999). Thus, if the BCR-activated Ba2+-permeable pathway were mediated by plasma membrane cation channels conformationally coupled to IP3R, then the D2477A IP3R-3 mutant would be expected to gate the coupled Ba2+-permeable receptor-activated channel in the plasma membrane despite its inability to release calcium from the stores. However, only the D2477E mutant, with intact gating and permeation properties, was able to restore both the release and the cation entry pathways driven by BCR stimulation.
Fig. 7. The D2477A mutant of the rat IP3R-3 does not restore BCR-dependent activation of Ba2+ entry in Ca2+-depleted, IP3R-KO DT40 cells. Fura-2-loaded IP3R-KO DT40 cells transfected with either the wild type or the D2477A mutant of the rat IP3R-3 were incubated in nominally Ca2+-free medium and then exposed to 2 µM thapsigargin. After complete store depletion, Ba2+ (10 mM) was added to the medium where indicated, and 2 min later anti-IgM (5 µg/ml) was added. Solid line, wild-type IP3R; dashed line, D2477A IP3R. Representative traces from three independent experiments are shown.
Discussion
WTDT40 B lymphocytes co-express three subtypes of IP3R: IP3R-1, -2 and -3 (Sugawara et al., 1997), which functionally interact to produce the oscillatory Ca2+ pattern observed with BCR activation by antigen- or antibody-dependent crosslinking (Miyakawa et al., 1999). A variant of these cells has been generated in which the genes coding for the three IP3R isoforms have been disrupted by homologous recombination (Sugawara et al., 1997). Such IP3R-KO cells respond to PLC-coupled receptors with generation of IP3, but they do not generate PLC-linked cytosolic Ca2+ signals. It has been shown previously that store-operated Ca2+ entry occurs normally in both wild-type and IP3R-KO DT40 cells following passive depletion of endogenous stores with thapsigargin (Sugawara et al., 1997; Broad et al., 2001; Ma et al., 2001). This endogenous store-operated pathway is highly selective for Ca2+ and exhibits minimal permeability to Ba2+ or Sr2+ (Figure 2A; see also Vazquez et al., 2001; Venkatachalam et al., 2001). Consistent with this conclusion, which is based on fluorescence measurements, Prakriya and Lewis have recently demonstrated that the store-operated entry in DT40 involves the highly selective calcium current, Icrac (Prakriya and Lewis, 2001).
The results of the current study demonstrate the presence of a novel mechanism for BCR-activation of divalent cation entry across the plasma membrane of B cells that requires fully functional IP3R, and apparently does not involve a conformational coupling mechanism. The most likely scenario is that in DT40 B lymphocytes, stimulation of the BCR-associated signaling machinery results in generation of IP3, which, in addition to inducing calcium release from stores through activation of endoplasmic reticulum-located IP3R, also activates plasma membrane located IP3R. Ca2+ entry through this pathway may contribute to BCR-mediated Ca2+ signaling, or, possibly, cation fluxes may modify store-operated Ca2+ entry in other ways. For example, if substantial cation fluxes were to result in membrane depolarization, then store-operated entry might actually be inhibited. This would be consistent with the observation of Hashimoto et al. (2001), who, using a membrane potential-sensitive dye, observed membrane depolarization and inhibition of CCE by BCR activation in DT40 cells. Thus, it would be useful to demonstrate the presence of an agonist-activated membrane current with properties expected of an IP3R. However, probably due to the small size of this current, we have not as yet succeeded in identifying a non-capacitative whole-cell current activated by agonist in either WTDT40 or IP3R-3 transfected IP3R-KO cells.
Although not the major focus of this study, the experiments involving transfection of IP3R-KO cells with IP3R-3 revealed interesting patterns of Ca2+ signaling in the release phase as well. Miyakawa et al. (1999) generated different combinations of single IP3R-KO DT40 cells and evaluated the contribution of each of the IP3R subtypes normally expressed in these cells (IP3R-1, -2 and -3) to the oscillatory Ca2+ pattern observed upon BCR activation. Interestingly, those studies suggested that only mutants expressing the IP3R-2 exhibited BCR-dependent Ca2+ oscillations comparable to the wild-type cells, whereas mutants expressing either IP3R-1 or IP3R-3 responded with a monophasic Ca2+ transient. In the present studies, we observed that transient expression of the rat IP3R-3 in IP3R-KO DT40 cells is sufficient to restore an oscillatory pattern for the Ca2+ response to BCR stimulation (26 of 42 EYFP+ cells). As differences in the expression level of the IP3R isoforms may affect the Ca2+ signaling pattern, it is possible that transient overexpression of the IP3R-3 could account for such a discrepancy. Our observations are also inconsistent with the suggestion from Hagar et al. (1998) that cells expressing predominantly type 3 IP3R would not support Ca2+ oscillations due to a lack of Ca2+-dependent inhibition of the channel. In this context, the ability of the IP3R-KO cells transiently and solely expressing the rat IP3R-3 to respond to BCR stimulation with clearly defined cytosolic Ca2+ oscillations could be taken as indirect evidence for biphasic regulation of the IP3R-3 upon cytoplasmic Ca2+ concentration; however, it is also possible that cyclical modulation of PLC activity could underlie these oscillations.
As discussed above, a number of publications have raised the issue of plasma membrane IP3R, but, with the exception of invertebrate olfactory neurons, the significance of these observations is unclear (Fasolato et al., 1994). Confusion undoubtedly arises because in some instances, IP3 appears to be capable of regulating store-operated channels (Vaca and Kunze, 1995; Kiselyov et al., 1998; Zubov et al., 1999); this probably represents a conformational coupling mechanism, rather than the presence of plasma membrane IP3R. In the current study, however, the use of Ba2+ as a surrogate for Ca2+, as well as findings presented in Figures 3–5, clearly demonstrate that DT40 B cells express a novel entry pathway that is distinct from the store-operated pathway, does not appear to involve conformational coupling and is dependent on the presence of a cation-permeable IP3R. Taken together with previous immunological evidence for the presence of IP3R in the B cell plasma membrane (Khan et al., 1996), it seems likely that this IP3R-dependent pathway reflects Ba2+ entry through plasma membrane IP3R cation channels.
Non-CCE mechanisms have been described in a variety of cell types (Fasolato et al., 1993; Byron and Taylor, 1995; Shuttleworth and Thompson, 1996; Carroll and Peralta, 1998; Broad et al., 1999). One of the hallmarks of non-capacitative pathways is their greater permeability of Ca2+ surrogates, as compared with Ca2+ (Byron and Taylor, 1995). In at least two different cell types, the receptor-activated, non-capacitative Ca2+ entry can be activated by arachidonic acid (Shuttleworth, 1997; Broad et al., 1999), leading to speculation that a receptor-regulated phospholipase A2 may be involved. However, in HEK293 cells, we previously found (Luo et al., 2001) that the non-capacitative Ca2+ entry activated by low concentrations of muscarinic agonists was inhibited by 2-aminoethoxydiphenyl borane, a compound that inhibits both store-operated channels, as well as IP3R (Maruyama et al., 1997; Braun et al., 2001; Broad et al., 2001; Dobrydneva and Blackmore, 2001; Gregory et al., 2001; Iwasaki et al., 2001; Ma et al., 2001; Prakriya and Lewis, 2001). It is thus tempting to speculate that plasma membrane IP3R may underlie, or at least contribute to, non-capacitative Ca2+ entry in cell types other than the B lymphocyte. This issue should be the focus of investigation in future studies in a variety of Ca2+-regulated systems.
Materials and methods
Cell culture, transfection and measurement of intracellular calcium
The immortalized chicken B lymphocyte cell line, DT40 (RIKEN Cell Bank No. RCB1464), and its mutant variant lacking all three IP3R types (RIKEN Cell Bank No. RCB1467) were kindly provided by Dr Tomohiro Kurosaki (Department of Molecular Genetics, Kansai Medical University). Cell culture and handling for intracellular calcium measurements using the calcium-sensitive dye Fura-2 were as described previously (Vazquez et al., 2001).
DT40 cells were transiently transfected by electroporation (300 V, 500 µF) with either the rat wild-type IP3R-3 in pCB6+ (provided by Dr Graeme Bell, University of Chicago, Chicago, IL), its mutants (see below) or vector alone (mock-transfected cells), along with pEYFP-C1 (Clontech, Palo Alto, CA) as a transfection marker. In some experiments, cells were transfected with the human M5 muscarinic receptor (kindly provided by Dr Lutz Birnbaumer, National Institutes of Environmental and Health Sciences, Research Triangle Park, NC); the corresponding cDNA was subcloned into an expression vector (lcf201, provided by Drs Jean-Marie Buerstedde/Hiroshi Arakawa, Heinrich-Pette-Institute, University of Hamburg, Germany) under the control of the chicken β-actin promoter for expression in DT40 cells. Cells were assayed 18–30 h post-transfection. Fluorescence measurements were performed on EYFP+ cells selected by their green fluorescence when excited at 485 nm. The fluorescence intensity of multiple Fura-2-loaded DT40 cells was monitored with a CCD camera-based imaging system (Universal Imaging) mounted on a Zeiss Axiovert 35 inverted microscope equipped with a Zeiss 40× (1.3 NA) fluor objective. A Sutter Instruments filter changer enabled alternative excitation at 340 and 380 nm, while the emission fluorescence was monitored at 510 nm with a Paultek Imaging camera (model PC-20) equipped with a GenIISys intensifier (Dage-MTI, Inc.). The images of multiple cells collected at each excitation wavelength were subsequently processed using the MetaFluor software (Universal Imaging Corp., West Chester, PA) to provide ratio images. In some experiments, responses of single cells were analyzed by utilizing a photomultiplier-based system, as described previously (Vazquez et al., 2001) (for example, Figures 1 and 3). Cells transfected with vector alone (mock-transfected cells) were used in parallel as controls. All experiments were performed at room temperature. The data are expressed as a ratio of Fura-2 fluorescence due to excitation at 340 nm to that due to excitation at 380 nm (F340/F380).
Mutagenesis
The cDNA of rat IP3R-3 (Blondel et al., 1993) in pCB6+ (kindly provided by Dr Graeme Bell) was used as a template for mutagenesis. D2477A and D2477E point mutations were generated using the QuikChange™ Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. Mutations were verified by DNA sequencing.
Immunocytochemistry for IP3R-3
DT40 cells attached to coverslips were fixed with 4% paraformaldehyde and permeabilized with 0.1% NP-40 in phosphate-buffered saline. Non-specific sites were blocked with 3% bovine serum albumin in TBST (Tris–HCl pH 8.0, 150 mM NaCl, 0.05% Tween-20) at room temperature for 30 min, and the cells were then incubated with anti-IP3R-3 monoclonal antibody (Transduction Laboratories, Lexington, KY) at 1:500 dilution. Secondary antibody was the Alexa fluor 488-labeled goat anti-mouse IgG antisera. The fluorescence images were acquired with a Zeiss LSM410 confocal laser scanning microscope (Carl Zeiss, Inc., Thornwood, NY) using an argon–krypton laser and excitation at 488 nm through a 40× (1.2 NA, water immersion) objective lens (optical slice thickness 1.5 µm).
Acknowledgments
Acknowledgements
We are grateful for Dr Lutz Birnbaumer for providing the human M5 muscarinic receptor cDNA, Dr Graeme Bell for the rat IP3R-3 cDNA, Dr Tomohiro Kurosaki for the wild-type and IP3R-KO DT40 cells, and Drs Jean-Marie Buerstedde and Hiroshi Arakawa for the lcf201 expression vector.
References
- Baba T.W., Giroir,B.P. and Humphries,E.H. (1985) Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology, 144, 139–151. [DOI] [PubMed] [Google Scholar]
- Barritt G.J. (1999) Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J., 337, 153–169. [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1995) Capacitative calcium entry. Biochem. J., 312, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. and Ehrlich,B.E. (1994) Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: Conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol., 104, 821–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. and Ehrlich,B.E. (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol., 145, 205–216. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda,J., Janssen,H., Seino,S. and Bell,G.I. (1993) Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract and other tissues. J. Biol. Chem., 268, 11356–11363. [PubMed] [Google Scholar]
- Boehning D. and Joseph,S.K. (2000) Functional properties of recombinant type I and type III inositol 1,4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J. Biol. Chem., 275, 21492–21499. [DOI] [PubMed] [Google Scholar]
- Braun F.-J., Broad,L.M., Armstrong,D.L. and Putney,J.W.,Jr (2001) Stable activation of single CRAC-channels in divalent cation-free solutions. J. Biol. Chem., 276, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Broad L.M., Cannon,T.R. and Taylor,C.W. (1999) A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J. Physiol. (Lond.), 517, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad L.M., Braun,F.-J., Lièvremont,J.-P., Bird,G.,St J., Kurosaki,T. and Putney,J.W.,Jr (2001) Role of the phospholipase C–inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current (Icrac) and capacitative calcium entry. J. Biol. Chem., 276, 15945–15952. [DOI] [PubMed] [Google Scholar]
- Buerstedde J.-M. and Takeda,S. (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell, 67, 179–188. [DOI] [PubMed] [Google Scholar]
- Bush K.T., Stuart,R.O., Li,S., Moura,L.A., Sharp,A.H., Ross,C.A. and Nigam,S.K. (1994) Epithelial inositol 1,4,5-trisphosphate receptors. Multiplicity of localization, solubility and isoforms. J. Biol. Chem., 269, 23694–23699. [PubMed] [Google Scholar]
- Byron K.L. and Taylor,C.W. (1995) Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. J. Physiol. (Lond.), 485, 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R.C. and Peralta,E.G. (1998) The m3 muscarinic acetylcholine receptor differentially regulates calcium influx and release through modulaton of monovalent cation channels. EMBO J., 17, 3036–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A.M., Ryugo,D.K., Sharp,A.H., Reed,R.R., Snyder,S.H. and Ronnett,G.V. (1993) Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience, 57, 339–352. [DOI] [PubMed] [Google Scholar]
- Dobrydneva Y. and Blackmore,P. (2001) 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol. Pharmacol., 60, 541–552. [PubMed] [Google Scholar]
- Doyle D.A., Cabral,J.M., Pfuetzner,R.A., Kuo,A., Gulbis,J.M., Cohen,S.L., Chait,B.T. and MacKinnon,R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science, 280, 69–76. [DOI] [PubMed] [Google Scholar]
- El-Daher S.S. et al. (2000) Distinct localization and function of 1,4,5IP3 receptor subtypes and the 1,3,4,5IP4 receptor GAP1IP4BP in highly purified human platelet membranes. Blood, 95, 3412–3422. [PubMed] [Google Scholar]
- Fadool D.A. and Ache,B.W. (1994) Inositol 1,3,4,5-tetrakisphosphate-gated channels interact with inositol 1,4,5-trisphosphate-gated channels in olfactory receptor neurons. Proc. Natl Acad. Sci. USA, 91, 9471–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Hoth,M., Matthews,G. and Penner,R. (1993) Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc. Natl Acad. Sci. USA, 90, 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Innocenti,B. and Pozzan,T. (1994) Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol. Sci., 15, 77–83. [DOI] [PubMed] [Google Scholar]
- Feng L. and Kraus-Friedmann,N. (1993) Association of the hepatic IP3 receptor with the plasma membrane: relevance to mode of action. Am. J. Physiol., 265, C1588–C1596. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Nakade,S., Miyawaki,A., Mikoshiba,K. and Ogawa,K. (1992) Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J. Cell Biol., 119, 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J., Munsch,J.A., Lam,T.H., Catlin,M.C., Costa,L.G., Molinski,T.F. and Pessah,I.N. (1997) Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron, 19, 723–733. [DOI] [PubMed] [Google Scholar]
- Gregory R.B., Rychkov,G. and Barritt,G.J. (2001) Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem. J., 354, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette G., Balla,T., Baukal,A.J. and Catt,K.J. (1988) Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in hepatic plasma membrane fraction. J. Biol. Chem., 263, 4541–4548. [PubMed] [Google Scholar]
- Hagar R.E., Burgstahler,A.D., Nathanson,M.H. and Ehrlich,B.E. (1998) Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature, 396, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A., Hirose,K., Kurosaki,T. and Iino,M. (2001) Negative control of store-operated Ca2+ influx by B cell receptor cross-linking. J. Immunol., 166, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Irvine R.F. (1990) ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates – a possible mechanism. FEBS Lett., 263, 5–9. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Mori,Y., Hara,Y., Uchida,K., Zhou,H. and Mikoshiba,K. (2001) 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels, 7, 429–439. [PubMed] [Google Scholar]
- Kashiwayanagi M. (1996) Dialysis of inositol 1,4,5-trisphosphate induces inward currents and Ca2+ uptake in frog olfactory receptor cells. Biochem. Biophys. Res. Commun., 225, 666–671. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Steiner,J.P., Klein,M.G., Schneider,M.F. and Snyder,S.H. (1992a) IP3 receptor: Localization to plasma membrane of T cells and cocapping with the T cell receptor. Science, 257, 815–818. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Steiner,J.P. and Snyder,S.H. (1992b) Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc. Natl Acad. Sci. USA, 89, 2849–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.A., Soloski,J.J., Sharp,A.H., Schilling,G., Sabatini,D.M., Li,S., Ross,C.A. and Snyder,S.H. (1996) Lymphocyte apoptosis: Mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science, 273, 503–507. [DOI] [PubMed] [Google Scholar]
- Kiselyov K., Xu,X., Mozhayeva,G., Kuo,T., Pessah,I., Mignery,G., Zhu,X., Birnbaumer,L. and Muallem,S. (1998) Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature, 396, 478–482. [DOI] [PubMed] [Google Scholar]
- Kiselyov K., Mignery,G.A., Zhu,M.X. and Muallem,S. (1999) The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol. Cell, 4, 423–429. [DOI] [PubMed] [Google Scholar]
- Lischka F.W., Zviman,M.M., Teeter,J.H. and Restrepo,D. (1999) Characterization of inositol-1,4,5-trisphosphate-gated channels in the plasma membrane of rat olfactory neurons. Biophys. J., 76, 1410–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Broad,L.M., Bird,G.,St J. and Putney,J.W.,Jr (2001) Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J. Biol. Chem., 276, 5613–5621. [DOI] [PubMed] [Google Scholar]
- Ma H.-T., Venkatachalam,K., Li,H.-S., Montell,C., Kurosaki,T., Patterson,R.L. and Gill,D.L. (2001) Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J. Biol. Chem., 276, 18888–18896. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Kanaji,T., Nakade,S., Kanno,T. and Mikoshiba,K. (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem., 122, 498–505. [DOI] [PubMed] [Google Scholar]
- Mayrleitner M., Schäfer,R. and Fleischer,S. (1995) IP3 receptor purified from liver plasma membrane is an (1,4,5)IP3 activated and (1,3,4,5)IP4 inhibited calcium permeable ion channel. Cell Calcium, 17, 141–153. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda,A., Yamazawa,T., Hirose,K., Kurosaki,T. and Iino,M. (1999) Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J., 18, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger S.D., Gleeson,R.A., Aldrich,H.C., Rust,N.C., Ache,B.W. and Greenberg,R.M. (2000) Characterization of a phosphoinositide-mediated odor transduction pathway reveals plasma membrane localization of an inositol 1,4,5-trisphosphate receptor in lobster olfactory receptor neurons. J. Biol. Chem., 275, 20450–20457. [DOI] [PubMed] [Google Scholar]
- Okada Y., Teeter,J.H. and Restrepo,D. (1994) Inositol 1,4,5-trisphosphate-gated conductance in isolated rat olfactory neurons. J. Neurophysiol., 71, 595–602. [DOI] [PubMed] [Google Scholar]
- Parekh A.B. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Prakriya M. and Lewis,R.S. (2001) Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. (Lond.), 536, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J.W. Jr, (1986) A model for receptor-regulated calcium entry. Cell Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Putney J.W. Jr, (1997a) Capacitative Calcium Entry. Landes Biomedical Publishing, Austin, TX.
- Putney J.W. Jr, (1997b) The type 3 inositol 1,4,5-trisphosphate receptor and capacitative calcium entry. Cell Calcium, 21, 257–261. [DOI] [PubMed] [Google Scholar]
- Quinton T.M. and Dean,W.L. (1996) Multiple inositol 1,4,5-trisphosphate receptor isoforms are present in platelets. Biochem. Biophys. Res. Commun., 224, 740–746. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T.J. (1997) Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem., 271, 21720–21725. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T.J. and Thompson,J.L. (1996) Evidence for a non-capacitative Ca2+ entry during [Ca2+] oscillations. Biochem. J., 316, 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki,M., Takata,M. and Kurosaki,T. (1997) Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J., 16, 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A., Tojyo,Y. and Turner,R.J. (2000) Evidence that type I, II and III inositol 1,4,5-trisphosphate receptors can occur as integral plasma membrane proteins. J. Biol. Chem., 275, 27488–27493. [DOI] [PubMed] [Google Scholar]
- Vaca L. and Kunze,D.L. (1995) IP3-activated Ca2+ channels in the plasma membrane of cultured vascular endothelial cells. Am. J. Physiol., 269, C733–C738. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J.M. and Martonosi,A. (1971) Sarcoplasmic reticulum. XII. The interaction of 8-anilino-1-naphthalene sulfonate with skeletal muscle microsomes. Arch. Biochem. Biophys., 144, 99–106. [DOI] [PubMed] [Google Scholar]
- Vazquez G., Lièvremont,J.-P., Bird,G.,St J. and Putney,J.W.,Jr (2001) Trp3 forms both both inositol trisphosphate receptor-dependent and independent store-operated cation channels in DT40 avian B-lymphocytes. Proc. Natl Acad. Sci. USA, 98, 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Ma,H.-T., Ford,D.L. and Gill,D.L. (2001) Expression of functional receptor-coupled TRPC3 channels in DT40 triple receptor InsP3 knockout cells. J. Biol. Chem., 276, 33980–33985. [DOI] [PubMed] [Google Scholar]
- Vilen B.J., Famiglietti,S.J., Carbone,A.M., Kay,B.K. and Cambier,J.C. (1997) B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J. Immunol., 159, 231–243. [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. (2001) Crucial role of type 1, but not type 3, inositol 1,4,5-trisphosphate (IP3) receptors in IP3-induced Ca2+ release, capacitative Ca 2+ entry and proliferation of A7r5 vascular smooth muscle cells. Circ. Res., 88, 202–209. [DOI] [PubMed] [Google Scholar]
- Yamaguchi D.T., Green,J., Kleeman,C.R. and Muallem,S. (1989) Properties of the depolarization-activated calcium and barium entry in osteoblast-like cells. J. Biol. Chem., 264, 197–204. [PubMed] [Google Scholar]
- Zubov A.I., Kaznacheeva,E.V., Alexeeno,V.A., Kiselyov,K., Muallem,S. and Mozhayeva,G. (1999) Regulation of the miniature plasma membrane Ca2+ channel Imin by IP3 receptors. J. Biol. Chem., 274, 25983–25985. [DOI] [PubMed] [Google Scholar]