Abstract
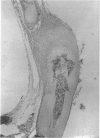
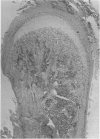
The mode of growth of the rat clavicle from 17 to 45 days of age was studied by means of vital staining (alizarin red S), histology and autoradiography (tritiated thymidine). In addition the clavicle on one side was subjected to periostomy at the age of 10 days and its length compared with that of its unoperated counterpart at the ages of 17 and 45 days. The alizarin red staining revealed that the medial end of the clavicle contributes to the length of the bone, while the lateral end appears to have mainly an articulatory function. Histologically, the medial end cartilage closely resembles the condylar cartilage of the mandible, whereas the lateral end appears to be composed of two cartilaginous structures separated by a mesenchymatous layer. Tritiated thymidine was deposited in the mesenchymal cells covering the medial end cartilage, whereas virtually no activity was observed in the mesenchyme of the lateral end cartilage. The periostomised clavicle was more slender in appearance than its control throughout the observation period. The two clavicles were of the same length at 17 days, but by 45 days the periostomised clavicle was significantly longer than the control. It is suggested that the growth of the clavicle is essentially comparable to the growth of the mandible. Length growth occurs in response to the action of the surrounding structures, while analogously to the mandibular condyle, the medial end cartilage actively translates the bone in a direction perpendicular to the articular surface, giving rise to its curved shape.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwood H. J. Growth of the mandibular condyle of the rat studied with tritiated thymidine. Arch Oral Biol. 1966 May;11(5):493–500. doi: 10.1016/0003-9969(66)90155-5. [DOI] [PubMed] [Google Scholar]
- Crilly R. G. Longitudinal overgrowth of chicken radius. J Anat. 1972 May;112(Pt 1):11–18. [PMC free article] [PubMed] [Google Scholar]
- Ellis E., 3rd, Carlson D. S. Histologic comparison of the costochondral, sternoclavicular, and temporomandibular joints during growth in Macaca mulatta. J Oral Maxillofac Surg. 1986 Apr;44(4):312–321. doi: 10.1016/0278-2391(86)90082-0. [DOI] [PubMed] [Google Scholar]
- Harkness E. M., Trotter W. D. Growth of transplants of rat humerus following circumferential division of the periosteum. J Anat. 1978 Jun;126(Pt 2):275–289. [PMC free article] [PubMed] [Google Scholar]
- Houghton G. R., Dekel S. The periosteal control of long bone growth. An experimental study in the rat. Acta Orthop Scand. 1979 Dec;50(6 Pt 1):635–637. doi: 10.3109/17453677908991285. [DOI] [PubMed] [Google Scholar]
- Koski K., Rönning O., Nakamura T. Periosteal control of mandibular condyle growth. Prog Clin Biol Res. 1985;187:413–423. [PubMed] [Google Scholar]
- McLain J. B., Vig P. S. Transverse periosteal sectioning and femur growth in the rat. Anat Rec. 1983 Oct;207(2):339–348. doi: 10.1002/ar.1092070213. [DOI] [PubMed] [Google Scholar]
- Petrovic A. G. Mechanisms and regulation of mandibular condylar growth. Acta Morphol Neerl Scand. 1972 Oct;10(1):25–34. [PubMed] [Google Scholar]
- Rönning O., Koski K. Letter: The effect of periostomy on the growth of the condylar process in the rat. Proc Finn Dent Soc. 1974 Feb;70(1):28–29. [PubMed] [Google Scholar]
- SCOTT J. H. The growth of the human face. Proc R Soc Med. 1954 Feb;47(2):91–100. doi: 10.1177/003591575404700203. [DOI] [PMC free article] [PubMed] [Google Scholar]