Abstract
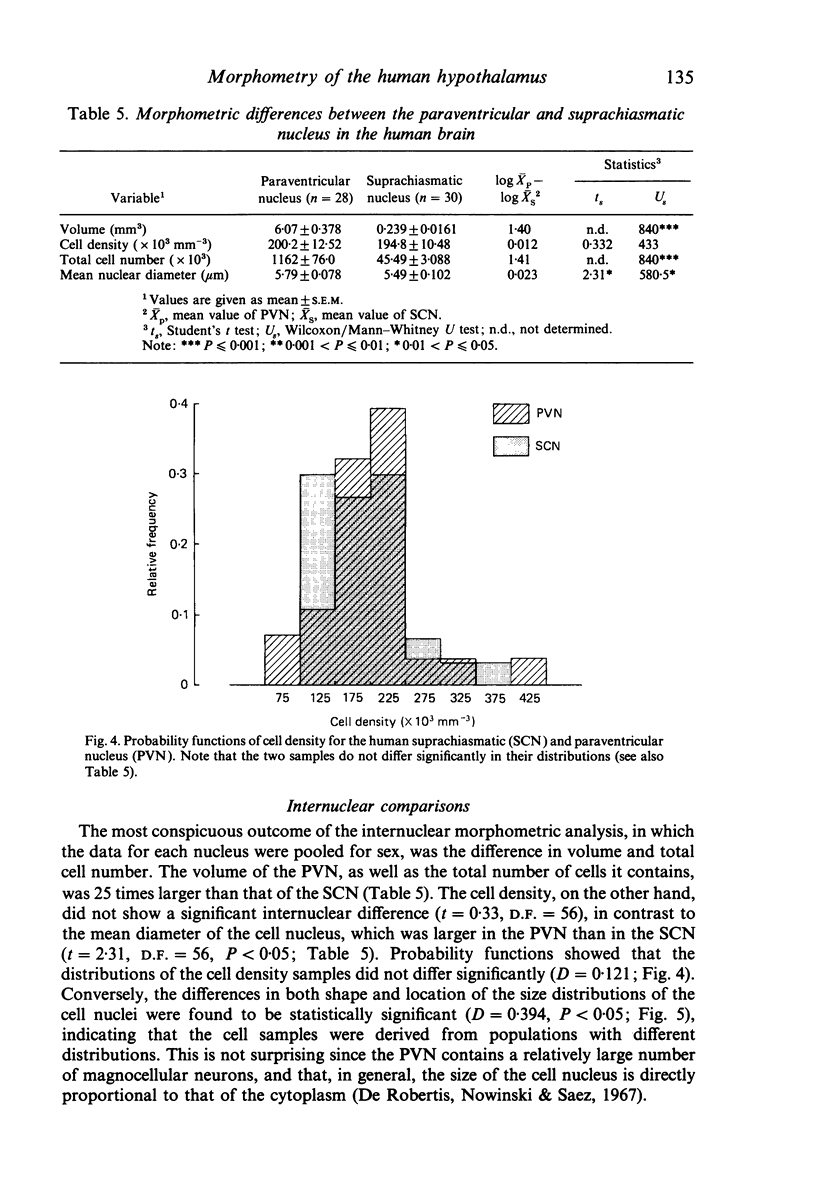
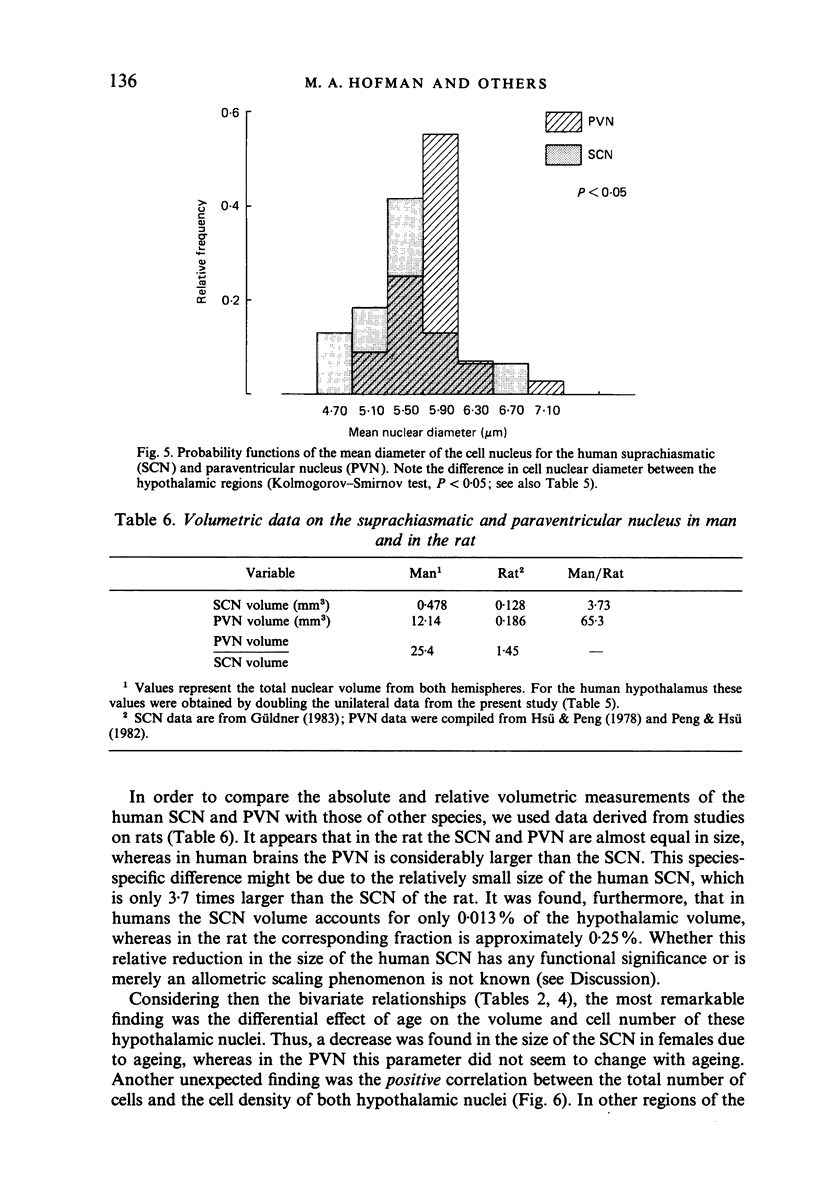
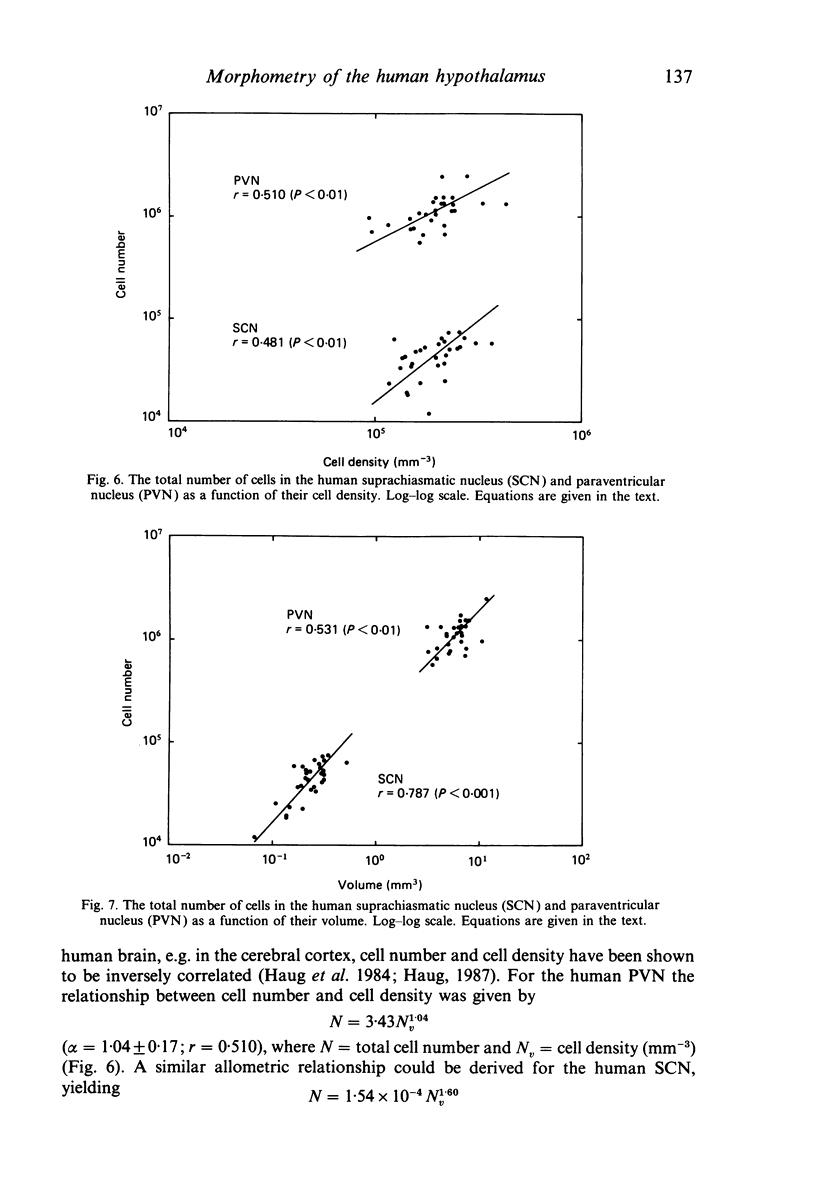
The size, shape and cellular morphology of the suprachiasmatic (SCN) and paraventricular nuclei (PVN) in the human hypothalamus were examined in relation to sex and age. In both nuclear regions the following parameters were determined: length of the rostrocaudal axis, maximum cross sectional area, volume, numerical cell density, total number of cells and the diameter of cell nuclei. No sexual difference was observed in any of these parameters, either in the SCN or in the PVN, with the exception of a sexual dimorphism in the shape of the SCN. In contrast to the absolute measurements, sexual differences were found in the internal structural organisation of these hypothalamic nuclei using multivariate regression analysis. Of the parameters measured only the volume of the SCN in females showed a continuous decrease with ageing, whereas the changes in the other variables were not consistent. Regression analysis revealed that this decrease in SCN volume is mainly caused by cell loss rather than by a reduction in cell size. Finally, a comparison of the volumetric measurements of the human SCN and PVN with those of the rat showed that the human SCN is reduced in size relative to other hypothalamic nuclei. Possible consequences of this phenomenon for the functional significance of the SCN in man are discussed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A. P., Gorski R. A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- BARGMANN W. Ueber die neurosekretorische Verknüpfung von Hypothalamus und Neurophypophyse. Z Zellforsch Mikrosk Anat. 1949;34(5):610–634. [PubMed] [Google Scholar]
- Bauchot R. Les modifications du poids encéphalique au cours de la fixation. J Hirnforsch. 1967;9(3):253–283. [PubMed] [Google Scholar]
- Berk M. L., Finkelstein J. A. Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience. 1981;6(8):1601–1624. doi: 10.1016/0306-4522(81)90227-x. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Swaab D. F., Dogterom J., van Leeuwen F. W. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978 Jan 31;186(3):423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Fliers E., Swaab D. F., Pool C. W., Verwer R. W. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 1985 Sep 2;342(1):45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Gordon J. H., Shryne J. E., Southam A. M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978 Jun 16;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Güldner F. H. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Exp Brain Res. 1983;50(2-3):373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- Güldner F. H. Sexual dimorphisms of axo-spine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neurosci Lett. 1982 Feb 12;28(2):145–150. doi: 10.1016/0304-3940(82)90143-4. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Orloff J. Antidiuretic hormone. Annu Rev Physiol. 1981;43:611–624. doi: 10.1146/annurev.ph.43.030181.003143. [DOI] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Am J Anat. 1987 Oct;180(2):126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Haug H., Kühl S., Mecke E., Sass N. L., Wasner K. The significance of morphometric procedures in the investigation of age changes in cytoarchitectonic structures of human brain. J Hirnforsch. 1984;25(4):353–374. [PubMed] [Google Scholar]
- Hines M., Davis F. C., Coquelin A., Goy R. W., Gorski R. A. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985 Jan;5(1):40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M. A. A biometric analysis of brain size in micrencephalics. J Neurol. 1984;231(2):87–93. doi: 10.1007/BF00313723. [DOI] [PubMed] [Google Scholar]
- Hofman M. A., Laan A. C., Uylings H. B. Bivariate linear models in neurobiology: problems of concept and methodology. J Neurosci Methods. 1986 Oct;18(1-2):103–114. doi: 10.1016/0165-0270(86)90114-7. [DOI] [PubMed] [Google Scholar]
- Holloway R. L., de Lacoste M. C. Sexual dimorphism in the human corpus callosum: an extension and replication study. Hum Neurobiol. 1986;5(2):87–91. [PubMed] [Google Scholar]
- Hsu H. K., Peng M. T. Hypothalamic neuron number of old female rats. Gerontology. 1978;24(6):434–440. doi: 10.1159/000212283. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Hauser H., Krey L. C. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977 Jul 22;197(4301):398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Le Blond C. B., Morris S., Karakiulakis G., Powell R., Thomas P. J. Development of sexual dimorphism in the suprachiasmatic nucleus of the rat. J Endocrinol. 1982 Oct;95(1):137–145. doi: 10.1677/joe.0.0950137. [DOI] [PubMed] [Google Scholar]
- Morton A. A quantitative analysis of the normal neuron population of the hypothalamic magnocellular nuclei in man and of their projections to the neurohypophysis. J Comp Neurol. 1969 Jun;136(2):143–157. doi: 10.1002/cne.901360203. [DOI] [PubMed] [Google Scholar]
- Peng M. T., Hsü H. K. No neuron loss from hypothalamic nuclei of old male rats. Gerontology. 1982;28(1):19–22. doi: 10.1159/000212507. [DOI] [PubMed] [Google Scholar]
- Perlow M. J., Reppert S. M., Tamarkin L., Wyatt R. J., Klein D. C. Photic regulation of the melatonin rhythm: monkey and man are not the same. Brain Res. 1980 Jan 20;182(1):211–216. doi: 10.1016/0006-8993(80)90848-3. [DOI] [PubMed] [Google Scholar]
- Pickard G. E. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol. 1982 Oct 10;211(1):65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- Pickard G. E., Turek F. W. Splitting of the circadian rhythm of activity is abolished by unilateral lesions of the suprachiasmatic nuclei. Science. 1982 Feb 26;215(4536):1119–1121. doi: 10.1126/science.7063843. [DOI] [PubMed] [Google Scholar]
- Pickard G. E., Turek F. W. The suprachiasmatic nuclei: two circadian clocks? Brain Res. 1983 Jun 6;268(2):201–210. doi: 10.1016/0006-8993(83)90486-9. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Perlow M. J., Ungerleider L. G., Mishkin M., Tamarkin L., Orloff D. G., Hoffman H. J., Klein D. C. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J Neurosci. 1981 Dec;1(12):1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. M., Fox T. O., Dikkes P., Pearlstein R. A. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res. 1986 Apr 23;371(2):380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., van Gool W. A., Swaab D. F., Hoogendijk J. E., Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987 Apr 21;409(2):259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982 Mar 1;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983 Aug 1;218(2):121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Fliers E., Partiman T. S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985 Sep 2;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J Comp Neurol. 1975 Mar 1;160(1):1–12. doi: 10.1002/cne.901600102. [DOI] [PubMed] [Google Scholar]
- Swanson L. W. Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977 Jun 10;128(2):346–353. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- Uylings H. B., van Eden C. G., Hofman M. A. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986 Oct;18(1-2):19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N., Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Res. 1979 Jan 12;160(2):307–326. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K., De Mey J. The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis. Cell Tissue Res. 1977 Jun 13;180(4):443–452. doi: 10.1007/BF00220167. [DOI] [PubMed] [Google Scholar]
- Watson R. E., Jr, Hoffmann G. E., Wiegand S. J. Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Res. 1986 Nov 19;398(1):157–163. doi: 10.1016/0006-8993(86)91261-8. [DOI] [PubMed] [Google Scholar]
- Zambrano D., De Robertis E. The effect of castration upon the ultrastructure of the rat hypothalamus. I. Supraoptic and paraventricular nuclei. Z Zellforsch Mikrosk Anat. 1968;86(4):487–498. doi: 10.1007/BF00324860. [DOI] [PubMed] [Google Scholar]
- Zilles K. Biometrische Analyse der Frischvolumina verschiedener prosencephaler Hirnregionen von 78 menschlichen, adulten Gehirnen. Gegenbaurs Morphol Jahrb. 1972;118(2):234–273. [PubMed] [Google Scholar]