Abstract
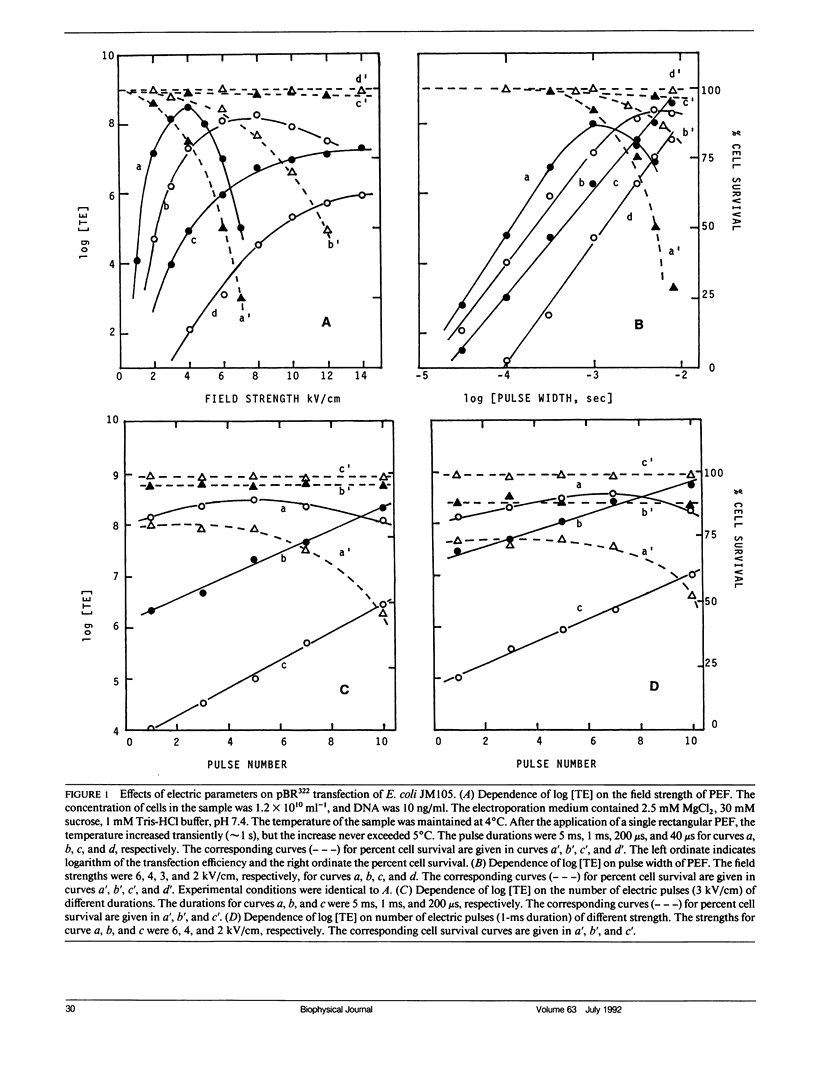
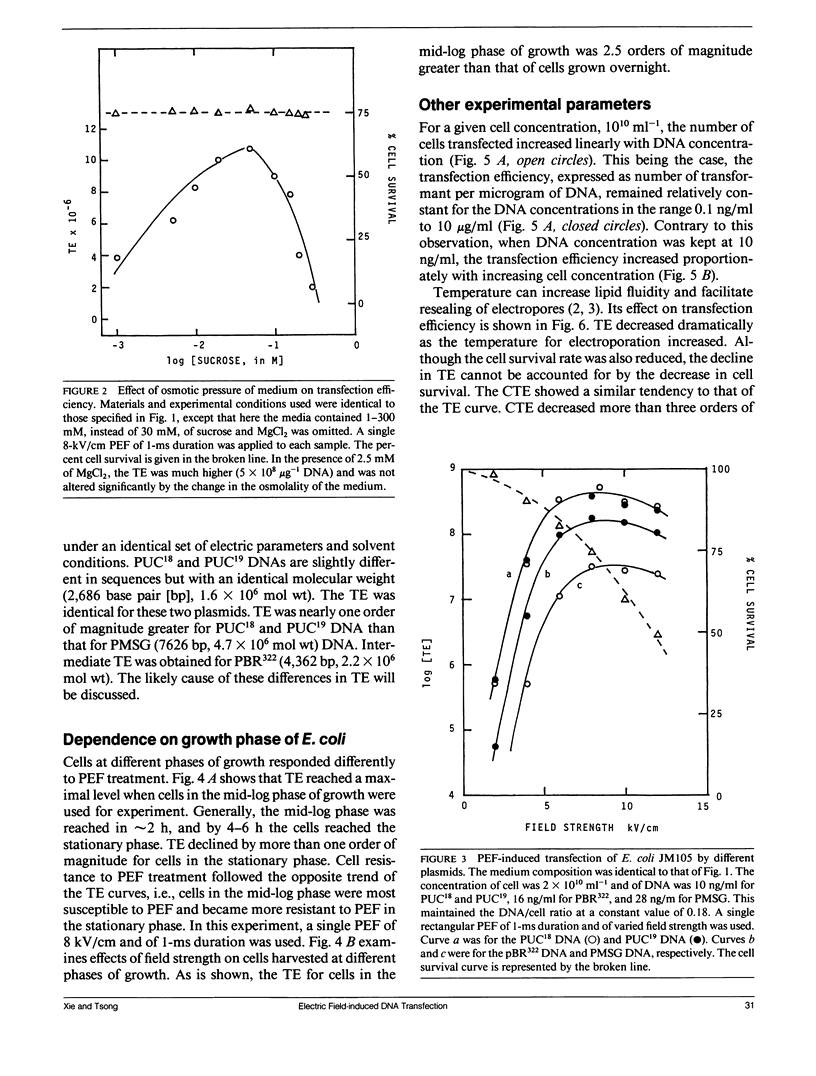
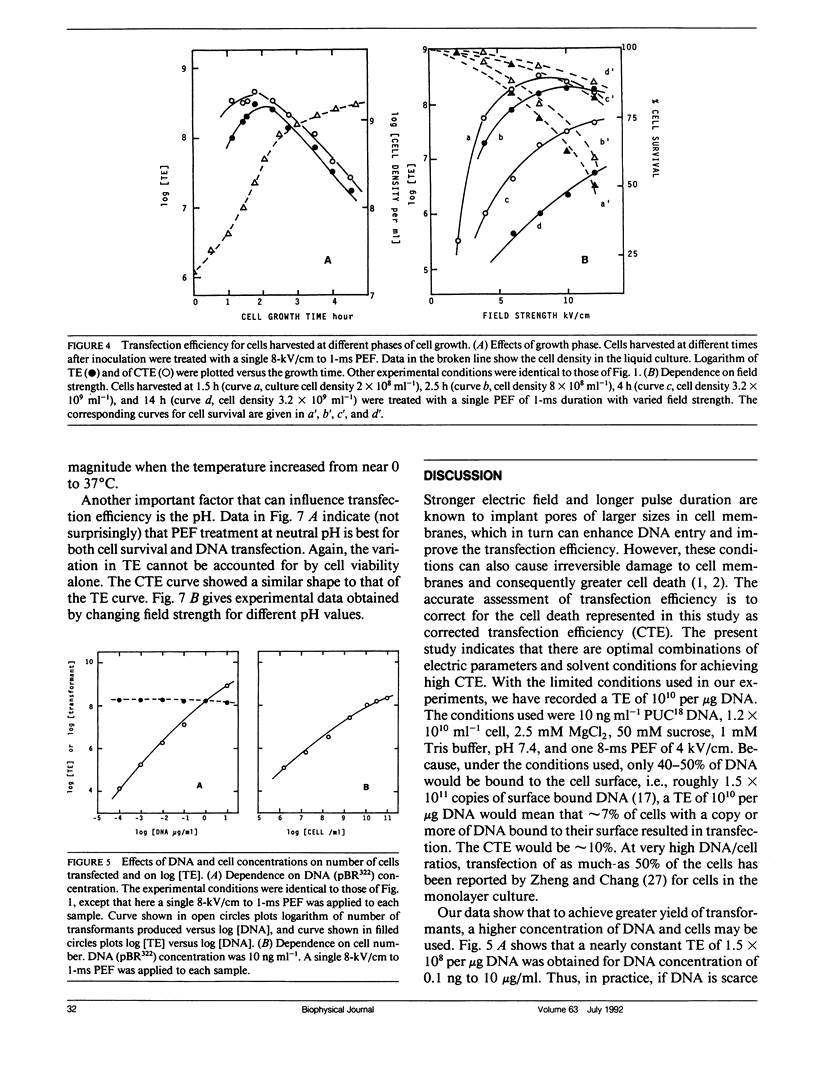
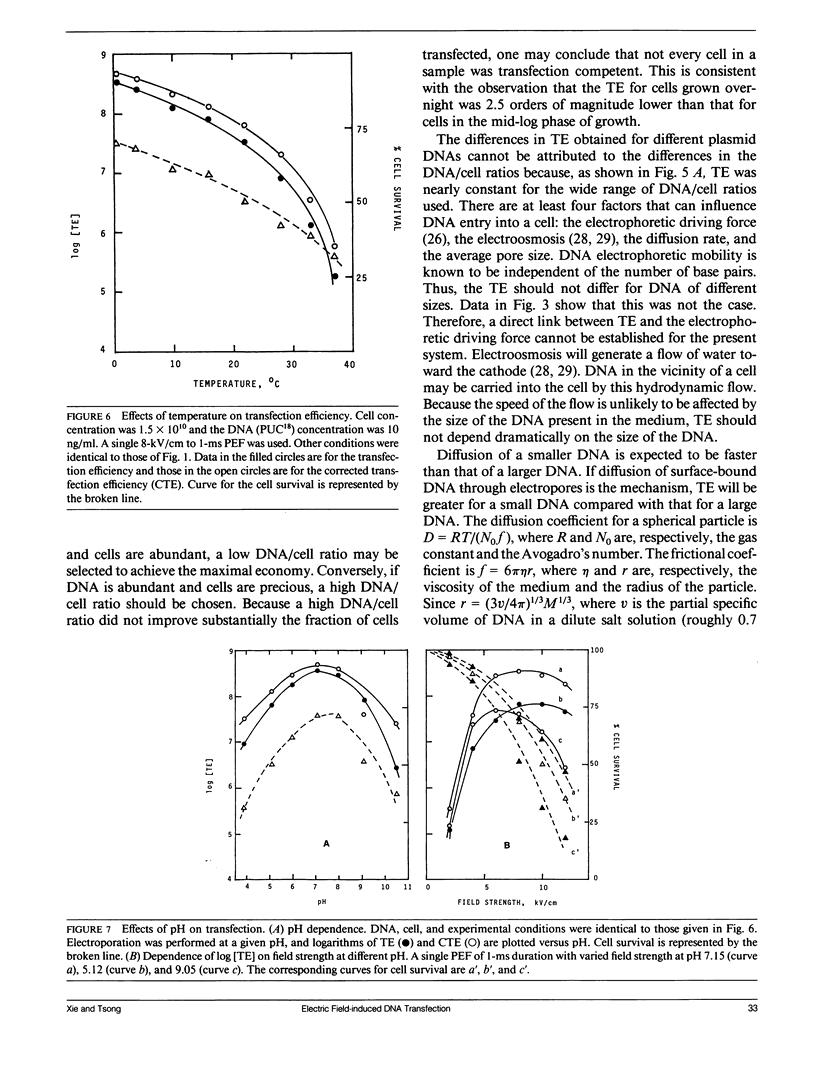
Electric parameters, osmolality, temperature, and pH of the suspending medium and the growth phase of cells, etc., are known to influence the efficiency of the pulsed electric field (PEF)-induced DNA transfection of cells. PEF-induced transfection of Escherichia coli JM105 by plasmid DNA PUC18, PUC19, PBR322, and PMSG has been used as a model system to establish quantitative relationships between these parameters and transfection efficiency. The main findings are summarized for experiments using unipolar square wave PEF. (a) For a given field strength (up to 6 kV/cm), the transfection efficiency (TE) was linearly dependent on the pulse width (up to 1 ms). (b) When field strength is fixed, Log [TE] correlated with the number of pulses applied. Similarly, when field duration was fixed, Log [TE] correlated with the number of pulses. (c) In the absence of MgCl2, TE showed a maximal value at 50 mM sucrose and was reduced by several fold at lower and higher sucrose concentrations. Cell survival was nearly constant in the range 1-300 mM sucrose. (d) E. coli in the early and mid-exponential growth phases was more susceptible to PEF for DNA transfection than it was in the stationary phase. (e) For a given set of electric parameters, TE was the highest at neutral pH and was greatly reduced at acidic and alkaline pH. (f) Increasing the temperature from 0 to 37 degrees C resulted in the reduction of TE by three orders of magnitude. This could reflect a rapid shrinking of pores at higher temperatures. (g) TE was inversely proportional to the square of the size of the plasmid DNA. By adjusting the above parameters to optimize transfection, a TE of 1010 1microg-1 DNA (PUC18) has been recorded. Further improvement in percent cell transfection may be expected by a more exhaustive search of conditions than the present study has done.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer D., Brandner G., Bodemer W. Dielectric breakdown of the red blood cell membrane and uptake of SV 40 DNA and mammalian cell RNA. Naturwissenschaften. 1976 Aug;63(8):391–391. doi: 10.1007/BF00607946. [DOI] [PubMed] [Google Scholar]
- Calvin N. M., Hanawalt P. C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988 Jun;170(6):2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sokolov A. V., Budker V. G. Electrostimulated uptake of DNA by liposomes. Biochim Biophys Acta. 1990 May 9;1024(1):179–183. doi: 10.1016/0005-2736(90)90222-a. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas D. L., Wei M. D., Febbroriello P., Carson J. H., Loew L. M. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989 Dec;56(6):1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler S., Wirth R. Transformation of bacteria with plasmid DNA by electroporation. Anal Biochem. 1988 Apr;170(1):38–44. doi: 10.1016/0003-2697(88)90086-3. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Klenchin V. A., Sukharev S. I., Serov S. M., Chernomordik L. V., Chizmadzhev YuA Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991 Oct;60(4):804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Lee T. M., Turgeon R., Wu R. Expression of a foreign gene linked to either a plant-virus or a Drosophila promoter, after electroporation of protoplasts of rice, wheat, and sorghum. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6815–6819. doi: 10.1073/pnas.83.18.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Kinosita K., Jr, Tsong T. Y. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim Biophys Acta. 1985 Feb 14;812(3):779–785. doi: 10.1016/0005-2736(85)90272-x. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- Xie T. D., Sun L., Tsong T. Y. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys J. 1990 Jul;58(1):13–19. doi: 10.1016/S0006-3495(90)82349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. D., Tsong T. Y. Study of mechanisms of electric field-induced DNA transfection. II. Transfection by low-amplitude, low-frequency alternating electric fields. Biophys J. 1990 Oct;58(4):897–903. doi: 10.1016/S0006-3495(90)82434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q. A., Chang D. C. High-efficiency gene transfection by in situ electroporation of cultured cells. Biochim Biophys Acta. 1991 Jan 17;1088(1):104–110. doi: 10.1016/0167-4781(91)90158-i. [DOI] [PubMed] [Google Scholar]


