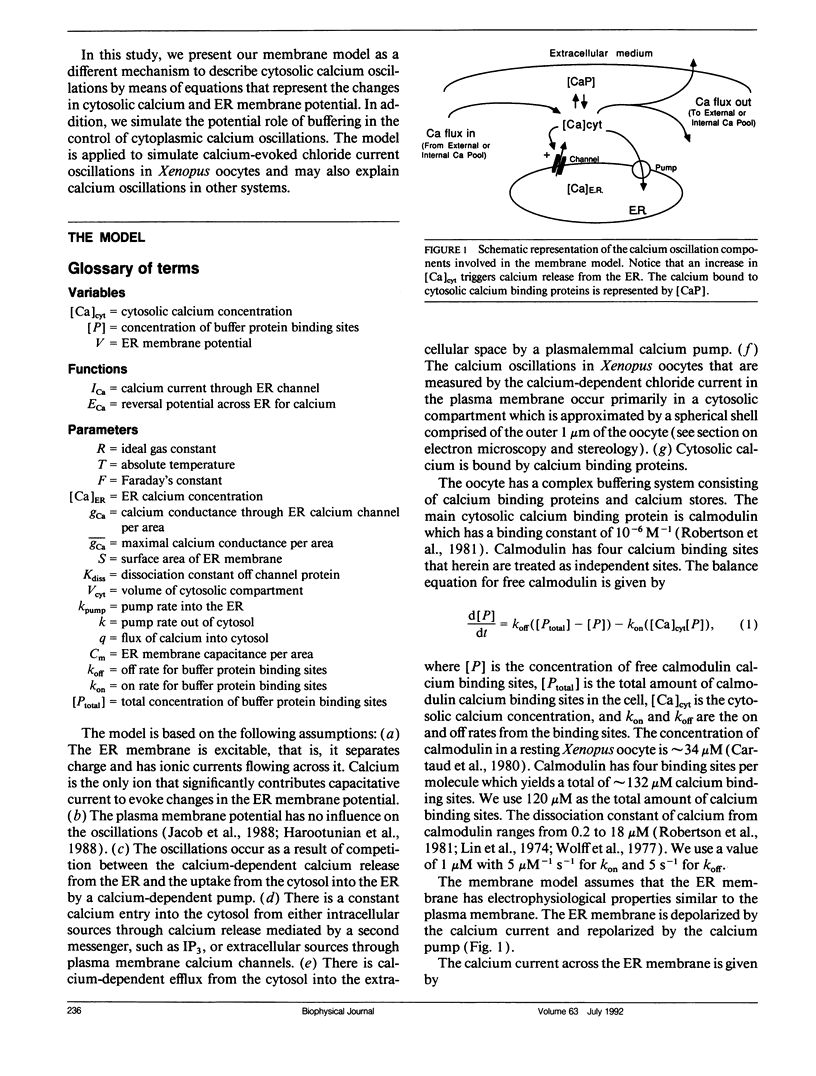
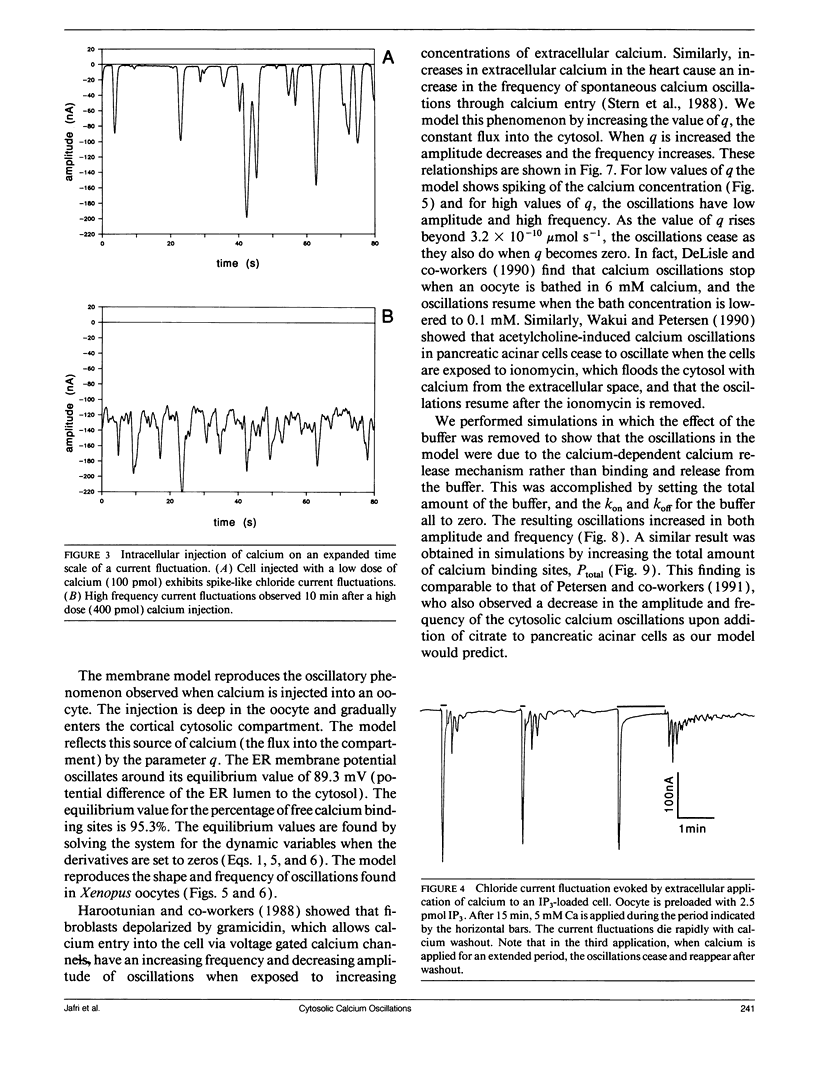
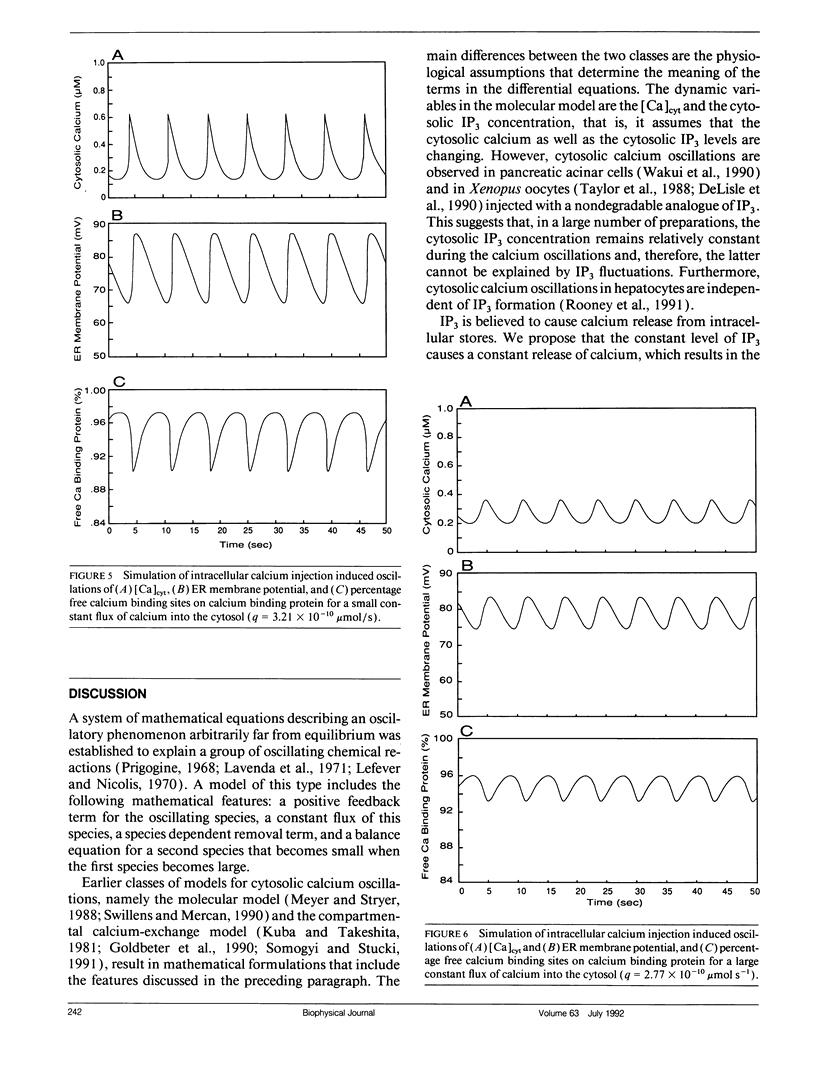
Abstract
Cytosolic calcium oscillations occur in a wide variety of cells and are involved in different cellular functions. We describe these calcium oscillations by a mathematical model based on the putative electrophysiological properties of the endoplasmic reticulum (ER) membrane. The salient features of our membrane model are calcium-dependent calcium channels and calcium pumps in the ER membrane, constant entry of calcium into the cytosol, calcium dependent removal from the cytosol, and buffering by cytoplasmic calcium binding proteins. Numerical integration of the model allows us to study the fluctuations in the cytosolic calcium concentration, the ER membrane potential, and the concentration of free calcium binding sites on a calcium binding protein. The model demonstrates the physiological features necessary for calcium oscillations and suggests that the level of calcium flux into the cytosol controls the frequency and amplitude of oscillations. The model also suggests that the level of buffering affects the frequency and amplitude of the oscillations. The model is supported by experiments indirectly measuring cytosolic calcium by calcium-induced chloride currents in Xenopus oocytes as well as cytosolic calcium oscillations observed in other preparations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler S. K., Poenie M., Tsien R. Y., Taylor P. Agonist-stimulated oscillations and cycling of intracellular free calcium in individual cultured muscle cells. J Biol Chem. 1988 Feb 5;263(4):1952–1959. [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cartaud A., Ozon R., Walsh M. P., Haiech J., Demaille J. G. Xenopus laevis oocyte calmodulin in the process of meiotic maturation. J Biol Chem. 1980 Oct 10;255(19):9404–9408. [PubMed] [Google Scholar]
- Charbonneau M., Grey R. D. The onset of activation responsiveness during maturation coincides with the formation of the cortical endoplasmic reticulum in oocytes of Xenopus laevis. Dev Biol. 1984 Mar;102(1):90–97. doi: 10.1016/0012-1606(84)90177-5. [DOI] [PubMed] [Google Scholar]
- Chay T. R., Keizer J. Minimal model for membrane oscillations in the pancreatic beta-cell. Biophys J. 1983 May;42(2):181–190. doi: 10.1016/S0006-3495(83)84384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Boton R. Interaction between injected Ca2+ and intracellular Ca2+ stores in Xenopus oocytes. FEBS Lett. 1990 Jul 2;267(1):22–24. doi: 10.1016/0014-5793(90)80278-q. [DOI] [PubMed] [Google Scholar]
- Dascal N., Gillo B., Lass Y. Role of calcium mobilization in mediation of acetylcholine-evoked chloride currents in Xenopus laevis oocytes. J Physiol. 1985 Sep;366:299–313. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Krause K. H., Denning G., Potter B. V., Welsh M. J. Effect of inositol trisphosphate and calcium on oscillating elevations of intracellular calcium in Xenopus oocytes. J Biol Chem. 1990 Jul 15;265(20):11726–11730. [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gandhi C. R., Ross D. H. Characterization of a high-affinity Mg2+-independent Ca2+-ATPase from rat brain synaptosomal membranes. J Neurochem. 1988 Jan;50(1):248–256. doi: 10.1111/j.1471-4159.1988.tb13257.x. [DOI] [PubMed] [Google Scholar]
- Gardiner D. M., Grey R. D. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J Cell Biol. 1983 Apr;96(4):1159–1163. doi: 10.1083/jcb.96.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B., Lass Y., Nadler E., Oron Y. The involvement of inositol 1,4,5-trisphosphate and calcium in the two-component response to acetylcholine in Xenopus oocytes. J Physiol. 1987 Nov;392:349–361. doi: 10.1113/jphysiol.1987.sp016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Tsien R. Y. Agonist-induced calcium oscillations in depolarized fibroblasts and their manipulation by photoreleased Ins(1,4,5)P3, Ca++, and Ca++ buffer. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):935–943. doi: 10.1101/sqb.1988.053.01.108. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Takeshita S. Simulation of intracellular Ca2+ oscillation in a sympathetic neurone. J Theor Biol. 1981 Dec 21;93(4):1009–1031. doi: 10.1016/0022-5193(81)90352-0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lakatta E. G., Capogrossi M. C., Kort A. A., Stern M. D. Spontaneous myocardial calcium oscillations: overview with emphasis on ryanodine and caffeine. Fed Proc. 1985 Dec;44(15):2977–2983. [PubMed] [Google Scholar]
- Lavenda B., Nicolis G., Herschkowitz-Kaufman M. Chemical instabilities and relaxation oscillations. J Theor Biol. 1971 Aug;32(2):283–292. doi: 10.1016/0022-5193(71)90166-4. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lefever R., Nicolis G. Chemical instabilities and sustained oscillations. J Theor Biol. 1971 Feb;30(2):267–284. doi: 10.1016/0022-5193(71)90054-3. [DOI] [PubMed] [Google Scholar]
- Lin Y. M., Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Purification, characterization, and active form of the protein activator from bovine brain. J Biol Chem. 1974 Aug 10;249(15):4943–4954. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T. Calcium channel and calcium pump involved in oscillatory hyperpolarizing responses of L-strain mouse fibroblasts. J Physiol. 1982 Jun;327:449–461. doi: 10.1113/jphysiol.1982.sp014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Activation of a common effector system by different brain neurotransmitter receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):37–45. doi: 10.1098/rspb.1987.0034. [DOI] [PubMed] [Google Scholar]
- Petersen C. C., Toescu E. C., Petersen O. H. Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J. 1991 Mar;10(3):527–533. doi: 10.1002/j.1460-2075.1991.tb07979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Renard D. C., Sass E. J., Thomas A. P. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991 Jul 5;266(19):12272–12282. [PubMed] [Google Scholar]
- Rooney T. A., Thomas A. P. Organization of intracellular calcium signals generated by inositol lipid-dependent hormones. Pharmacol Ther. 1991;49(3):223–237. doi: 10.1016/0163-7258(91)90056-r. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi R., Stucki J. W. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991 Jun 15;266(17):11068–11077. [PubMed] [Google Scholar]
- Stern M. D., Capogrossi M. C., Lakatta E. G. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988 Dec;9(5-6):247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Swillens S., Mercan D. Computer simulation of a cytosolic calcium oscillator. Biochem J. 1990 Nov 1;271(3):835–838. doi: 10.1042/bj2710835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Brown K. D., Cooke A. M., Potter B. V. DL-myo-inositol 1,4,5-trisphosphorothioate mobilizes intracellular calcium in Swiss 3T3 cells and Xenopus oocytes. Biochem Biophys Res Commun. 1988 Jan 29;150(2):626–632. doi: 10.1016/0006-291x(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Wakui M., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by acetylcholine or intracellular infusion of inositol trisphosphate or Ca2+ can be inhibited by internal Ca2+. FEBS Lett. 1990 Apr 24;263(2):206–208. doi: 10.1016/0014-5793(90)81374-w. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]