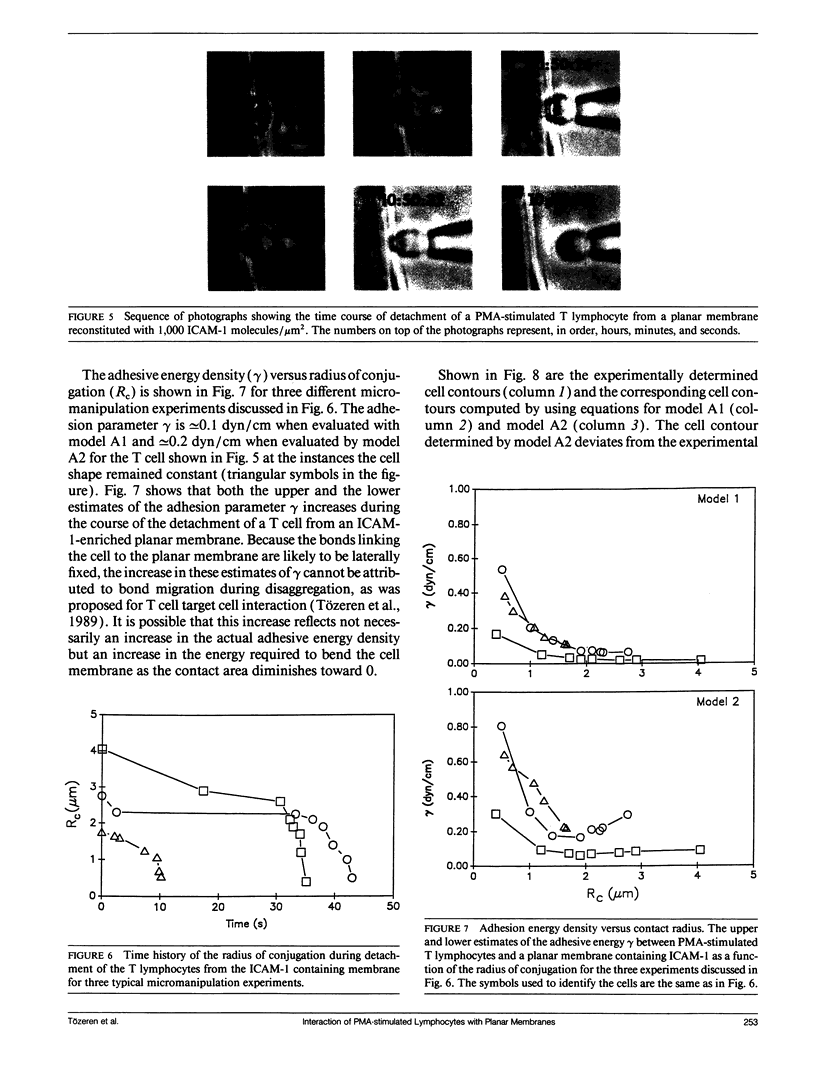
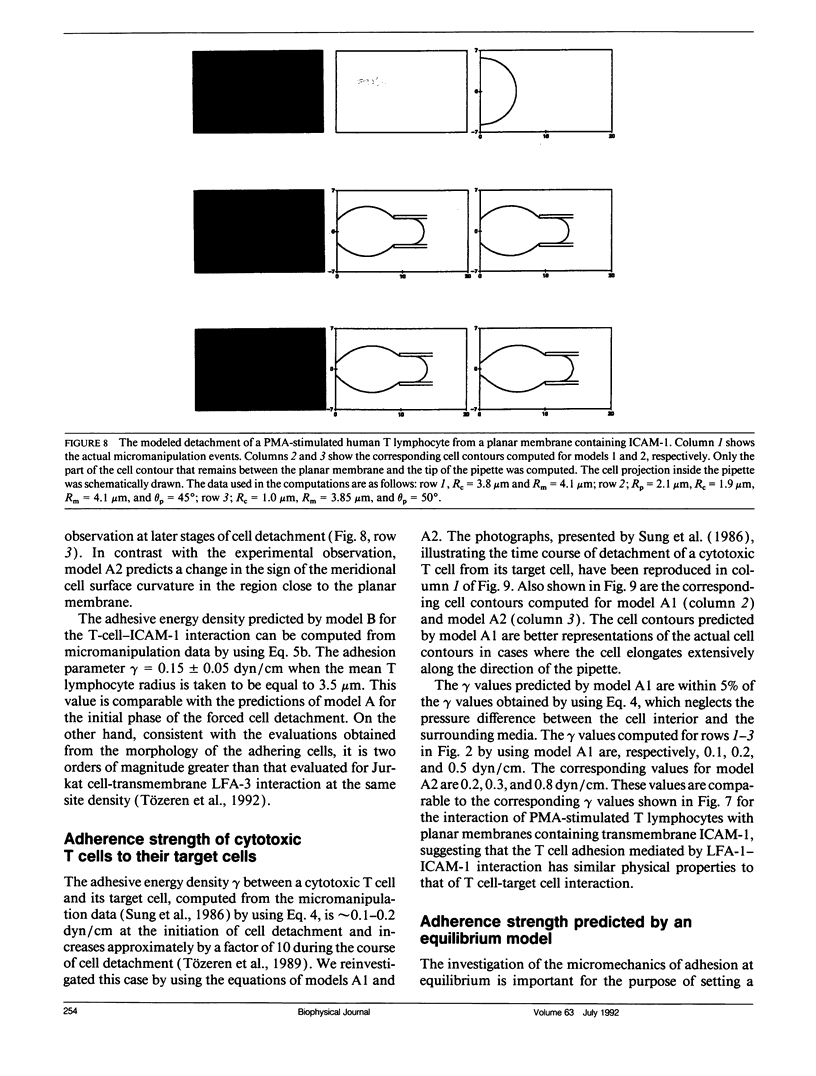
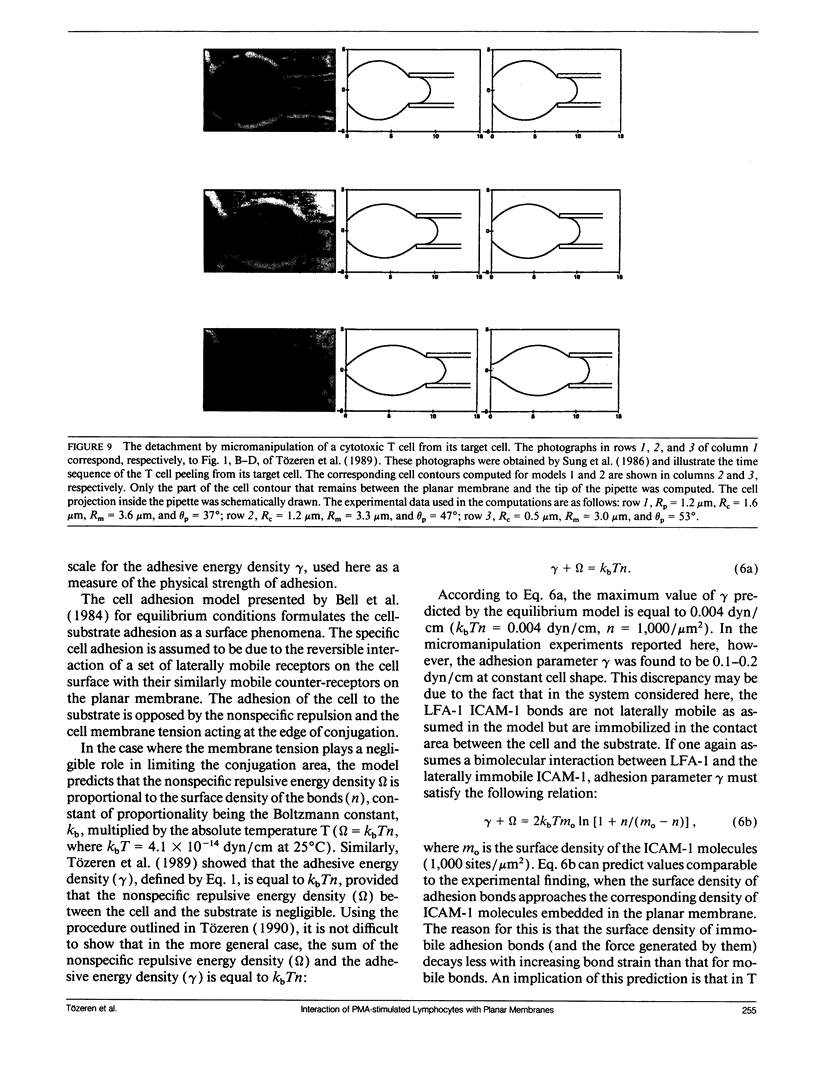
Abstract
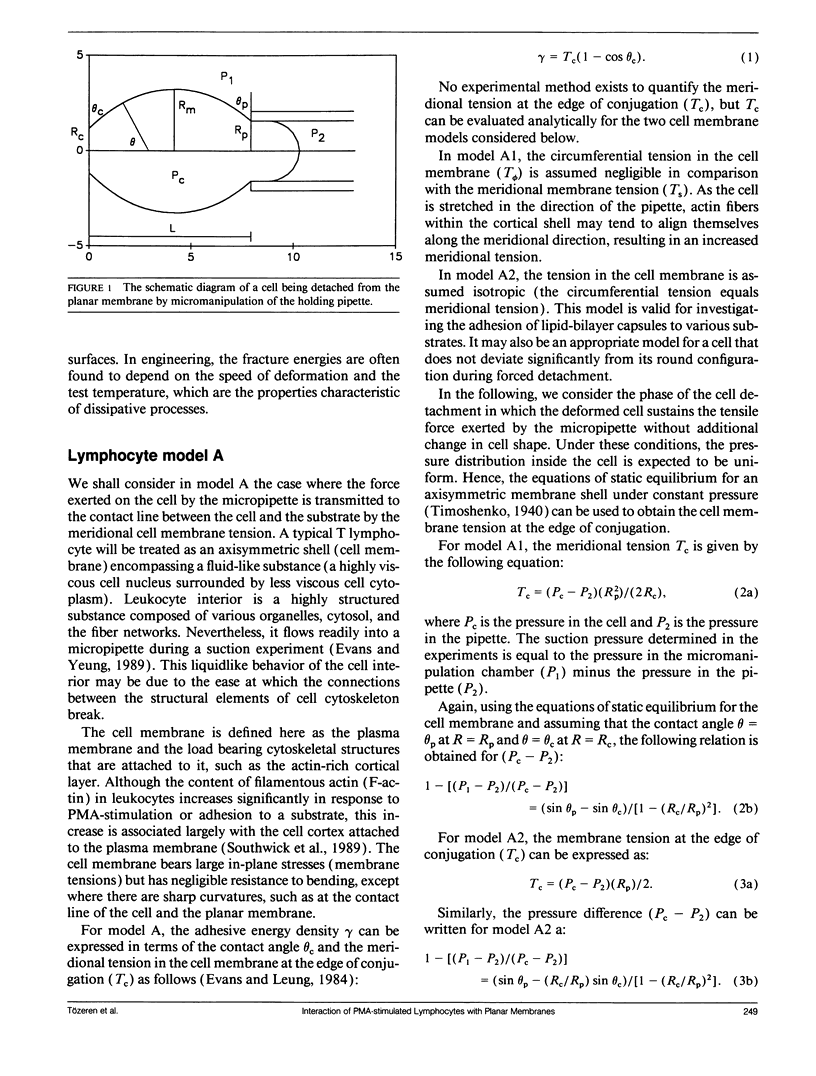
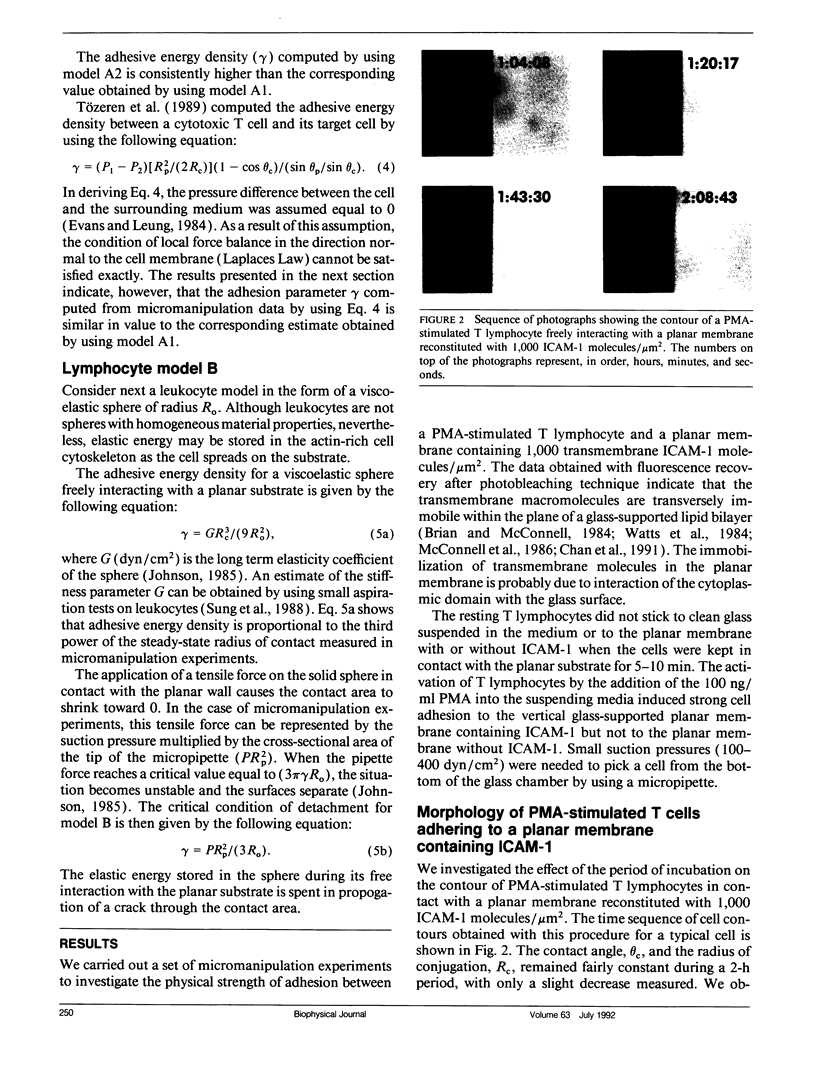
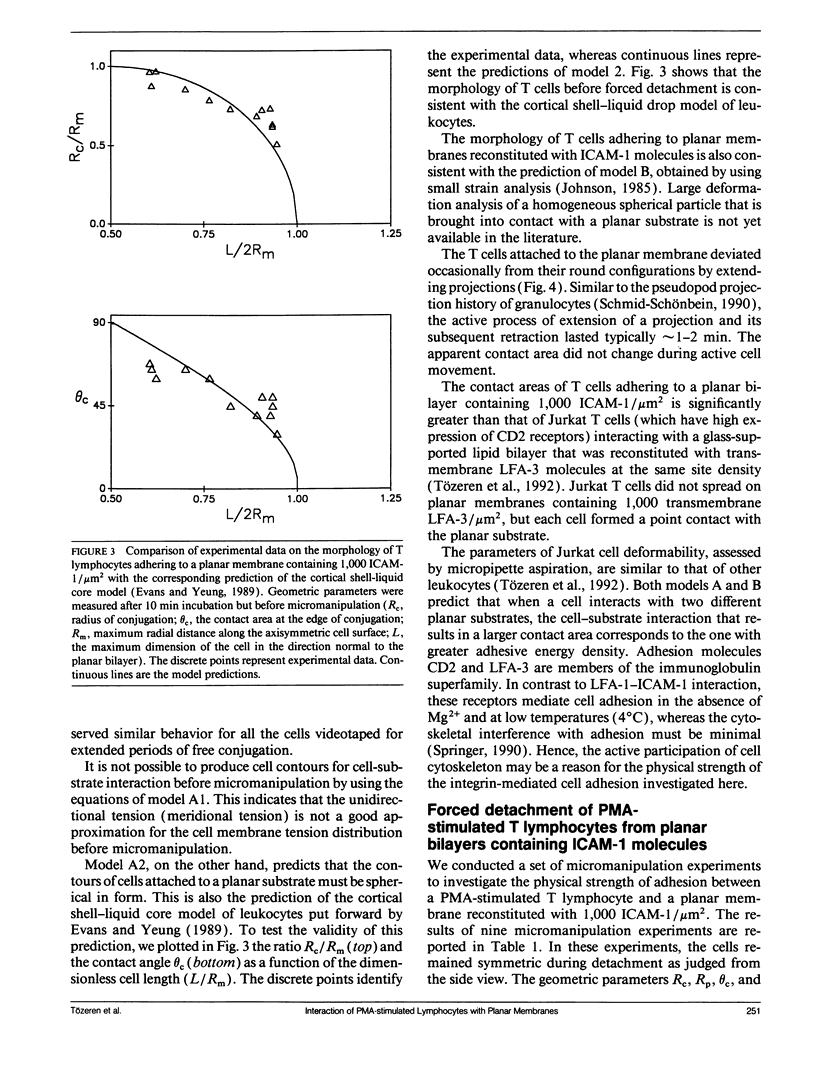
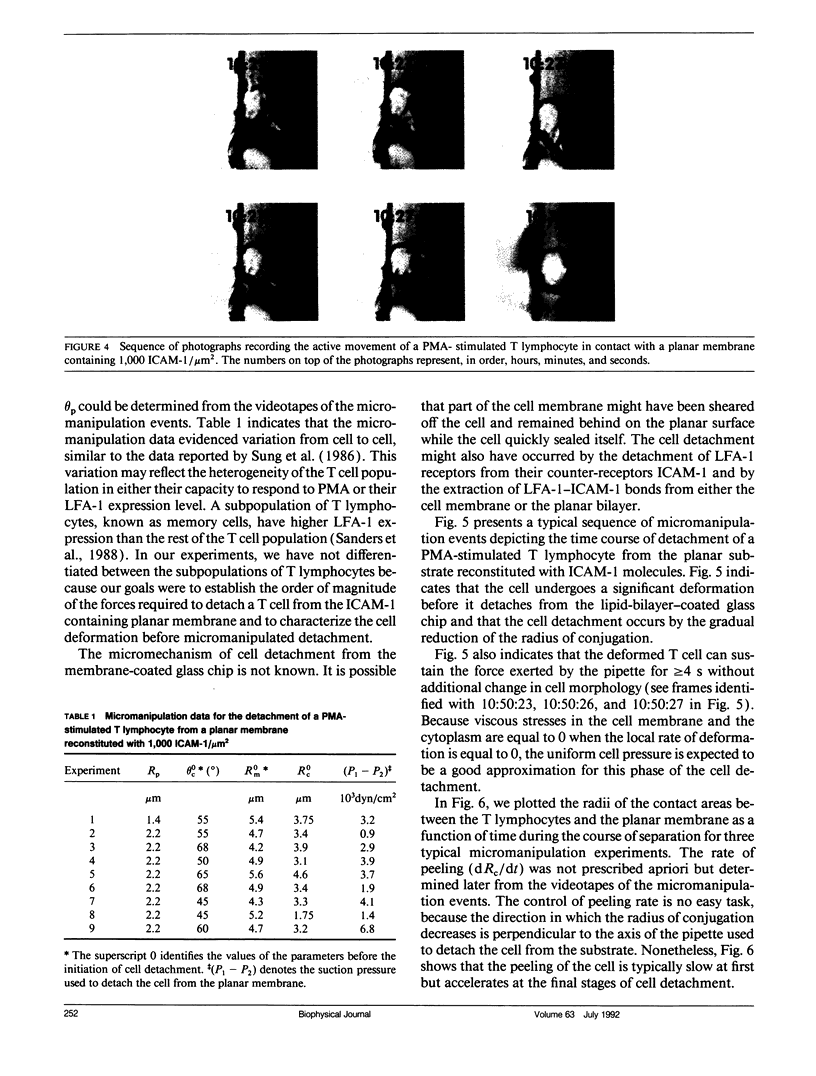
This paper presents an analytical and experimental methodology to determine the physical strength of cell adhesion to a planar membrane containing one set of adhesion molecules. In particular, the T lymphocyte adhesion due to the interaction of the lymphocyte function associated molecule 1 on the surface of the cell, with its counter-receptor, intercellular adhesion molecule-1 (ICAM-1), on the planar membrane, was investigated. A micromanipulation method and mathematical analysis of cell deformation were used to determine (a) the area of conjugation between the cell and the substrate and (b) the energy that must be supplied to detach a unit area of the cell membrane from its substrate. T lymphocytes stimulated with phorbol 12-myristate-13-acetate (PMA) conjugated strongly with the planar membrane containing purified ICAM-1. The T lymphocytes attached to the planar membrane deviated occasionally from their round configuration by extending pseudopods but without changing the size of the contact area. These adherent cells were dramatically deformed and then detached when pulled away from the planar membrane by a micropipette. Detachment occurred by a gradual decrease in the radius of the contact area. The physical strength of adhesion between a PMA-stimulated T lymphocyte and a planar membrane containing 1,000 ICAM-1 molecules/micron 2 was comparable to the strength of adhesion between a cytotoxic T cell and its target cell. The comparison of the adhesive energy density, measured at constant cell shape, with the model predictions suggests that the physical strength of cell adhesion may increase significantly when the adhesion bonds in the contact area are immobilized by the actin cytoskeleton.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian A. A., McConnell H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6159–6163. doi: 10.1073/pnas.81.19.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S. J. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chan P. Y., Lawrence M. B., Dustin M. L., Ferguson L. M., Golan D. E., Springer T. A. Influence of receptor lateral mobility on adhesion strengthening between membranes containing LFA-3 and CD2. J Cell Biol. 1991 Oct;115(1):245–255. doi: 10.1083/jcb.115.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Dong C., Skalak R., Sung K. L., Schmid-Schönbein G. W., Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988 Feb;110(1):27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Olive D., Springer T. A. Correlation of CD2 binding and functional properties of multimeric and monomeric lymphocyte function-associated antigen 3. J Exp Med. 1989 Feb 1;169(2):503–517. doi: 10.1084/jem.169.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Kalina M., Berke G. Contact regions of cytotoxic T lymphocyte-target cell conjugates. Cell Immunol. 1976 Jul;25(1):41–51. doi: 10.1016/0008-8749(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Phatak P. D., Packman C. H., Lichtman M. A. Protein kinase C modulates actin conformation in human T lymphocytes. J Immunol. 1988 Nov 1;141(9):2929–2934. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders V. M., Snyder J. M., Uhr J. W., Vitetta E. S. Characterization of the physical interaction between antigen-specific B and T cells. J Immunol. 1986 Oct 15;137(8):2395–2404. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Leukocyte biophysics. An invited review. Cell Biophys. 1990 Oct;17(2):107–135. doi: 10.1007/BF02990492. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Caulfield J. P., Michon J., Hein A., Kamada M. M., MacDermott R. P., Stevens R. L., Ritz J. T11/CD2 activation of cloned human natural killer cells results in increased conjugate formation and exocytosis of cytolytic granules. J Immunol. 1988 Feb 1;140(3):991–1002. [PubMed] [Google Scholar]
- Southwick F. S., Dabiri G. A., Paschetto M., Zigmond S. H. Polymorphonuclear leukocyte adherence induces actin polymerization by a transduction pathway which differs from that used by chemoattractants. J Cell Biol. 1989 Oct;109(4 Pt 1):1561–1569. doi: 10.1083/jcb.109.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Dynamic changes in viscoelastic properties in cytotoxic T-lymphocyte-mediated killing. J Cell Sci. 1988 Oct;91(Pt 2):179–189. doi: 10.1242/jcs.91.2.179. [DOI] [PubMed] [Google Scholar]
- Tozeren A. Cell-cell, cell-substrate adhesion: theoretical and experimental considerations. J Biomech Eng. 1990 Aug;112(3):311–318. doi: 10.1115/1.2891189. [DOI] [PubMed] [Google Scholar]
- Tozeren A., Sung K. L., Chien S. Theoretical and experimental studies on cross-bridge migration during cell disaggregation. Biophys J. 1989 Mar;55(3):479–487. doi: 10.1016/S0006-3495(89)82841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Sung K. L., Sung L. A., Dustin M. L., Chan P. Y., Springer T. A., Chien S. Micromanipulation of adhesion of a Jurkat cell to a planar bilayer membrane containing lymphocyte function-associated antigen 3 molecules. J Cell Biol. 1992 Feb;116(4):997–1006. doi: 10.1083/jcb.116.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A., Evans E. Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys J. 1989 Jul;56(1):139–149. doi: 10.1016/S0006-3495(89)82659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]







