Abstract
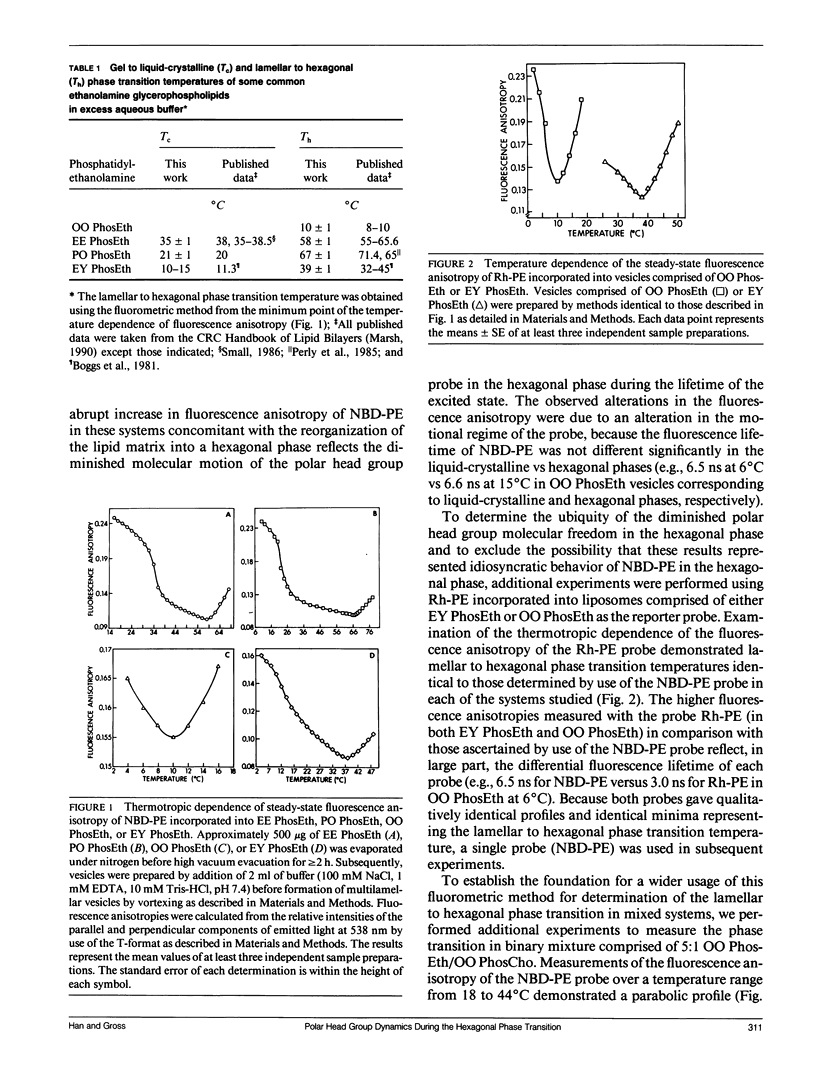
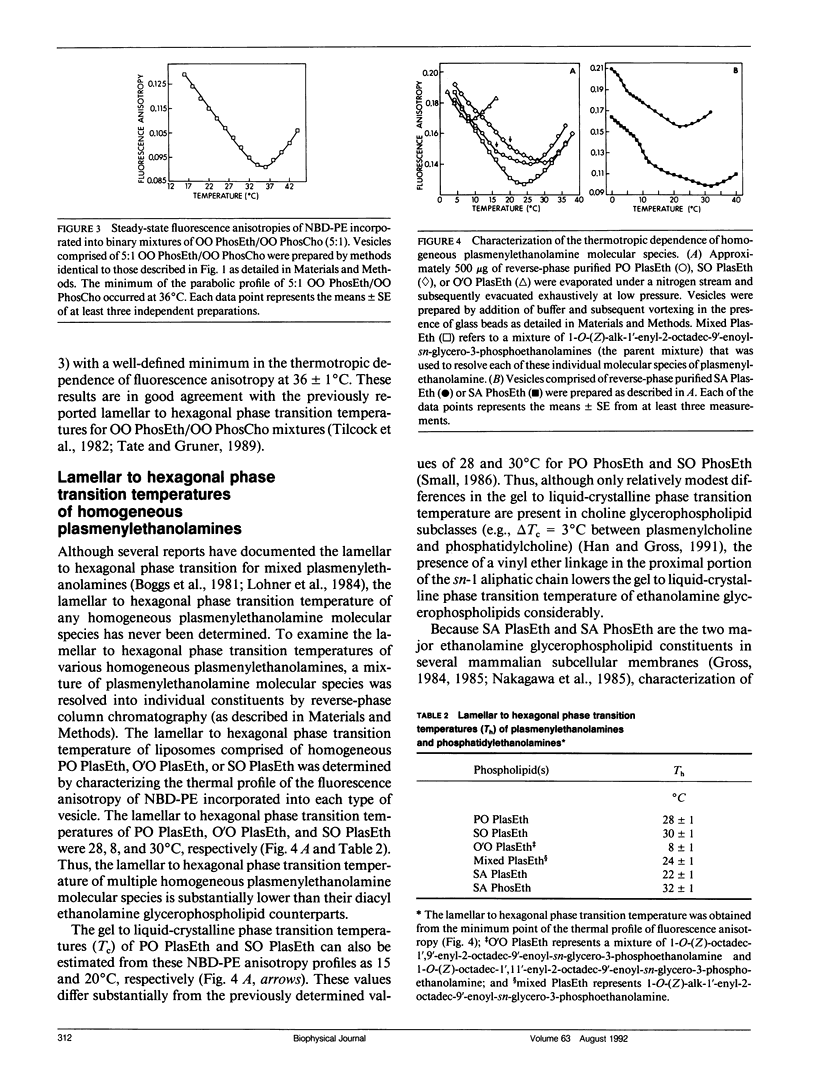
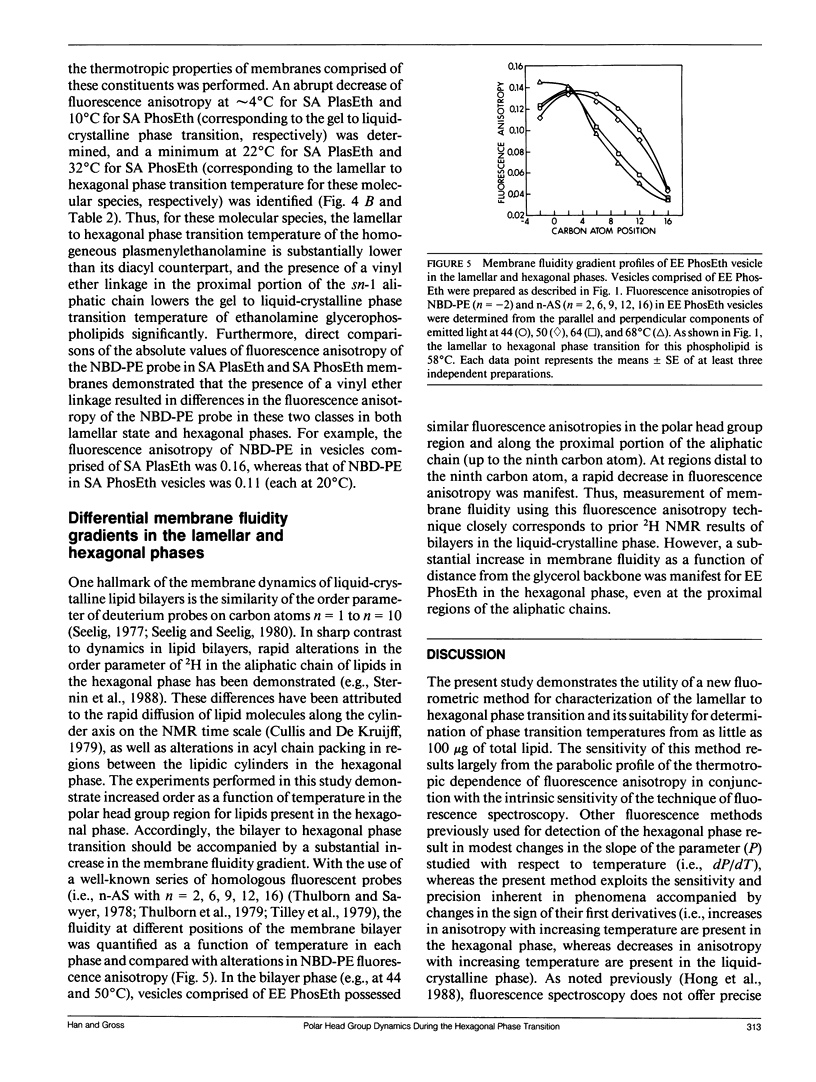
The temperature dependence of the fluorescence anisotropy of polar head group labeled fluorophores (i.e., N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)dipalmitoyl-L- alpha-phosphatidylethanolamine or N-(lissamine rhodamine B sulfonyl)dipalmitoyl-L-alpha-phosphatidylethanol- amine) incorporated into multiple phosphatidylethanolamine molecular species was parabolic, possessing minima (dr/dT = 0) that precisely correlated with the respective lamellar (L alpha) to hexagonal (HII) phase transition temperature of each species. The parabolic alterations in the thermotropic behavior of these fluorophores were due to increased motional constraints in the polar head group region during heating (dr/dT greater than 0), because significant alterations in the fluorescence lifetimes of these probes during the phase transition did not occur. The sensitivity inherent in identification of peak minima was exploited to determine the lamellar to hexagonal phase transition temperatures of several homogeneous molecular species of plasmenylethanolamine (e.g., the transition temperature of 1-O-(Z)-hexadec-1'-enyl-2-octadec-9'- enoyl-sn-glycero-3-phosphoethanolamine was 28 degrees C). Experiments using ethanolamine glycerophospholipids containing either an ester or a vinyl ether linkage at the sn-1 position demonstrated that introduction of the vinyl ether constituent increased the propensity of these species to assume the hexagonal phase. Collectively, these results identify and substantiate a new technique for the characterization of the lamellar to hexagonal phase transition in phospholipids that requires only small amounts of phospholipids present in dilute membrane suspensions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Stamp D., Hughes D. W., Deber C. M. Influence of ether linkage on the lamellar to hexagonal phase transition of ethanolamine phospholipids. Biochemistry. 1981 Sep 29;20(20):5728–5735. doi: 10.1021/bi00523a015. [DOI] [PubMed] [Google Scholar]
- Chen S. Y., Cheng K. H., Van der Meer B. W., Beechem J. M. Effects of lateral diffusion on the fluorescence anisotropy in hexagonal lipid phases. II. An experimental study. Biophys J. 1990 Dec;58(6):1527–1537. doi: 10.1016/S0006-3495(90)82497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H. Fluorescence depolarization study of lamellar liquid crystalline to inverted cylindrical micellar phase transition of phosphatidylethanolamine. Biophys J. 1989 Jun;55(6):1025–1031. doi: 10.1016/S0006-3495(89)82901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Leonard R., Tardieu A., Branton D. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta. 1970;219(1):47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. Destabilization of phosphatidylethanolamine liposomes at the hexagonal phase transition temperature. Biochemistry. 1986 Jan 28;25(2):285–294. doi: 10.1021/bi00350a001. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. Fusion of phosphatidylethanolamine-containing liposomes and mechanism of the L alpha-HII phase transition. Biochemistry. 1986 Jul 15;25(14):4141–4147. doi: 10.1021/bi00362a023. [DOI] [PubMed] [Google Scholar]
- Fink K. L., Gross R. W. Modulation of canine myocardial sarcolemmal membrane fluidity by amphiphilic compounds. Circ Res. 1984 Nov;55(5):585–594. doi: 10.1161/01.res.55.5.585. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Pluschke G., Overath P., Seelig J. Structure of Escherichia coli membranes. Fatty acyl chain order parameters of inner and outer membranes and derived liposomes. Biochemistry. 1980 Apr 15;19(8):1638–1643. doi: 10.1021/bi00549a018. [DOI] [PubMed] [Google Scholar]
- Gross R. W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984 Jan 3;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- Gross R. W. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry. 1985 Mar 26;24(7):1662–1668. doi: 10.1021/bi00328a014. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. L., Gross R. W. Alterations in membrane dynamics elicited by amphiphilic compounds are augmented in plasmenylcholine bilayers. Biochim Biophys Acta. 1991 Oct 14;1069(1):37–45. doi: 10.1016/0005-2736(91)90101-d. [DOI] [PubMed] [Google Scholar]
- Han X. L., Gross R. W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990 May 22;29(20):4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- Harlos K., Eibl H. Hexagonal phases in phospholipids with saturated chains: phosphatidylethanolamines and phosphatidic acids. Biochemistry. 1981 May 12;20(10):2888–2892. doi: 10.1021/bi00513a027. [DOI] [PubMed] [Google Scholar]
- Hong K., Baldwin P. A., Allen T. M., Papahadjopoulos D. Fluorometric detection of the bilayer-to-hexagonal phase transition in liposomes. Biochemistry. 1988 May 31;27(11):3947–3955. doi: 10.1021/bi00411a009. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Cherek H., Balter A. Correction of timing errors in photomultiplier tubes used in phase-modulation fluorometry. J Biochem Biophys Methods. 1981 Sep;5(3):131–146. doi: 10.1016/0165-022x(81)90012-9. [DOI] [PubMed] [Google Scholar]
- Larsson K. Lipid phase transitions in membranes involving intrinsic periodic curvature. Chem Phys Lipids. 1988 Nov;49(1-2):65–67. doi: 10.1016/0009-3084(88)90065-5. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Martin A., Cameron D. G. Characterization by infrared spectroscopy of the bilayer to nonbilayer phase transition of phosphatidylethanolamines. Biochemistry. 1981 May 26;20(11):3138–3145. doi: 10.1021/bi00514a024. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Sugiura T., Waku K. The molecular species composition of diacyl-, alkylacyl- and alkenylacylglycerophospholipids in rabbit alveolar macrophages. High amounts of 1-O-hexadecyl-2-arachidonyl molecular species in alkylacylglycerophosphocholine. Biochim Biophys Acta. 1985 Feb 8;833(2):323–329. [PubMed] [Google Scholar]
- Nieva J. L., Castresana J., Alonso A. The lamellar to hexagonal phase transition in phosphatidylethanolamine liposomes: a fluorescence anisotropy study. Biochem Biophys Res Commun. 1990 May 16;168(3):987–992. doi: 10.1016/0006-291x(90)91126-d. [DOI] [PubMed] [Google Scholar]
- Perly B., Smith I. C., Jarrell H. C. Effects of replacement of a double bond by a cyclopropane ring in phosphatidylethanolamines: a 2H NMR study of phase transitions and molecular organization. Biochemistry. 1985 Feb 12;24(4):1055–1063. doi: 10.1021/bi00325a038. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972 Feb 11;255(2):484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Sternin E., Fine B., Bloom M., Tilcock C. P., Wong K. F., Cullis P. R. Acyl chain orientational order in the hexagonal HII phase of phospholipid-water dispersions. Biophys J. 1988 Oct;54(4):689–694. doi: 10.1016/S0006-3495(88)83004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs C. D., Williams B. W., Boni L. T., Hoek J. B., Taraschi T. F., Rubin E. On the use of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)phosphatidylethanolamine in the study of lipid polymorphism. Biochim Biophys Acta. 1989 Nov 17;986(1):89–96. doi: 10.1016/0005-2736(89)90276-9. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Sawyer W. H. Properties and the locations of a set of fluorescent probes sensitive to the fluidity gradient of the lipid bilayer. Biochim Biophys Acta. 1978 Aug 4;511(2):125–140. doi: 10.1016/0005-2736(78)90308-5. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Tilley L. M., Sawyer W. H., Treloar F. E. The use of n-(9-anthroyloxy) fatty acids to determine fluidity and polarity gradients in phospholipid bilayers. Biochim Biophys Acta. 1979 Dec 4;558(2):166–178. doi: 10.1016/0005-2736(79)90057-9. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 1982 Sep 14;21(19):4596–4601. doi: 10.1021/bi00262a013. [DOI] [PubMed] [Google Scholar]
- Tilley L., Thulborn K. R., Sawyer W. H. An assessment of the fluidity gradient of the lipid bilayer as determined by a set of n-(9-anthroyloxy) fatty acids (n = 2, 6, 9, 12, 16). J Biol Chem. 1979 Apr 25;254(8):2592–2594. [PubMed] [Google Scholar]
- Van der Meer B. W., Cheng K. H., Chen S. Y. Effects of lateral diffusion on the fluorescence anisotropy in hexagonal lipid phases. I. Theory. Biophys J. 1990 Dec;58(6):1517–1526. doi: 10.1016/S0006-3495(90)82496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L. Lipid regulation of cell membrane structure and function. FASEB J. 1989 May;3(7):1833–1842. [PubMed] [Google Scholar]


