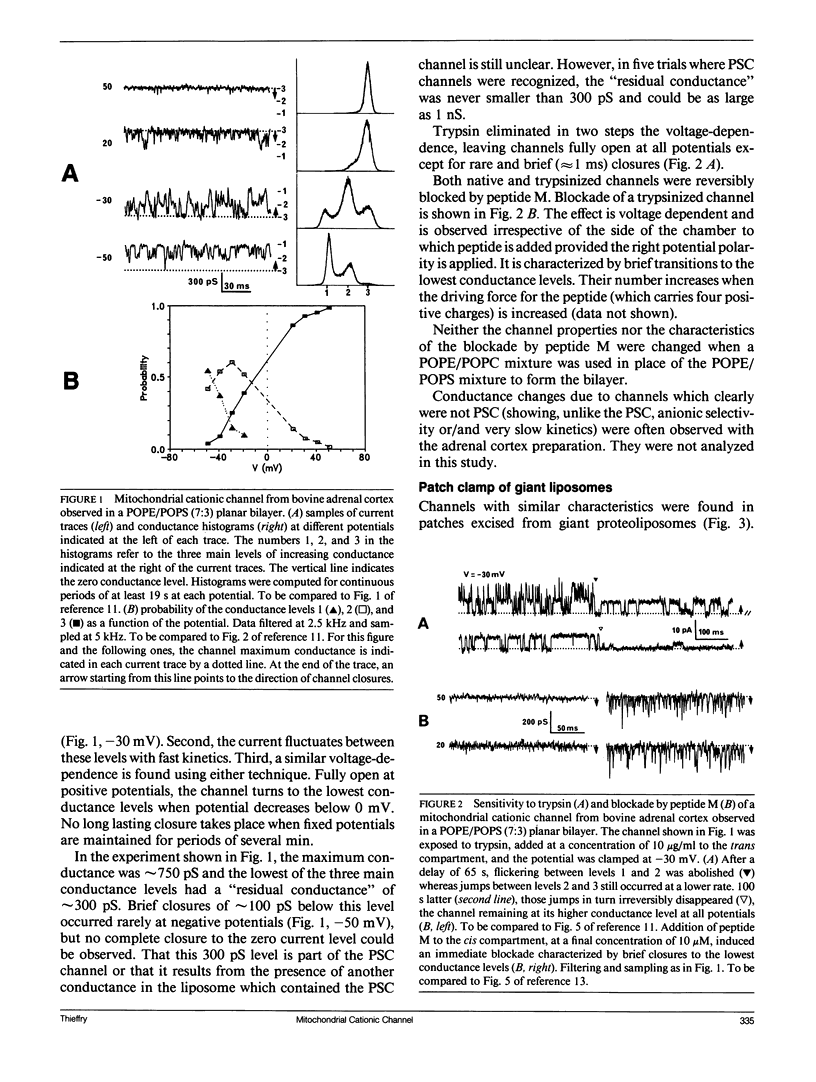
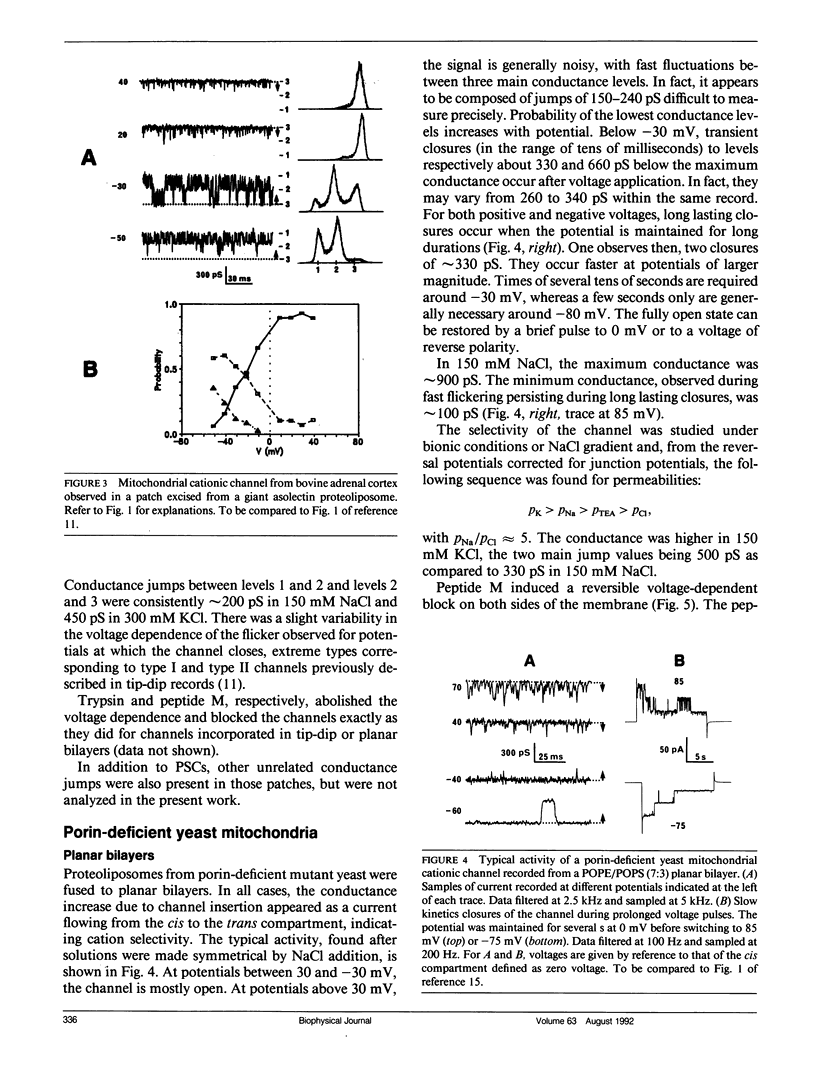
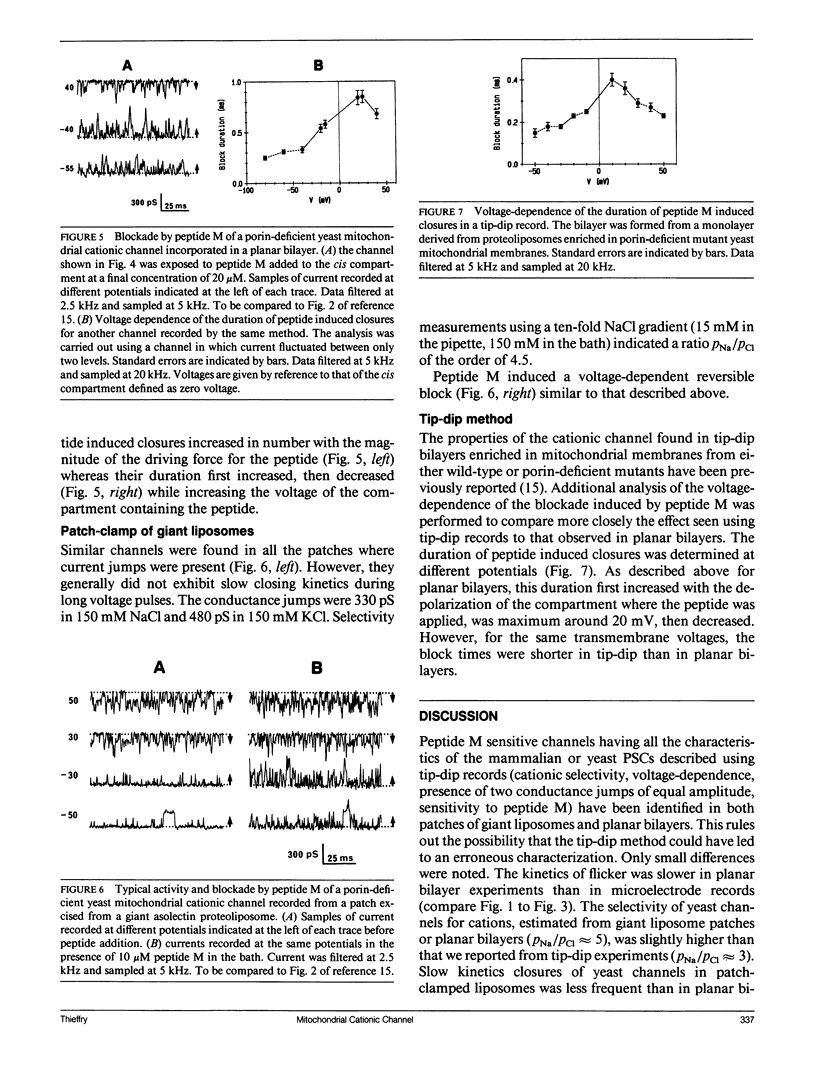
Abstract
A voltage-dependent cationic channel of large conductance is observed in phospholipid bilayers formed by the tip-dip method from proteoliposomes derived from mitochondrial membranes. It is blocked by peptide M, a 13 residue peptide having the properties of a mitochondrial signal sequence. To verify the reliability of the experimental approach, mitochondrial membranes from bovine adrenal cortex or porin-deficient mutant yeast were either fused to planar bilayers or incorporated in giant liposomes which were studied by patch clamp. Cationic channels were found with both techniques. They had the same conductance levels and voltage-dependence as those which have been described using the tip-dip method. Moreover, they were similarly blocked by peptide M. The voltage-dependence of block duration was analyzed in planar bilayer and tip-dip records. Results strengthen the idea that peptide M might cross the channel. Other mitochondrial channels were observed in planar bilayers and patch clamp of giant liposomes. Because they were never detected in tip-dip records, they are likely to be inactivated at the surface monolayer used to form the bilayer in this type of experiment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Schmid A., Dihanich M. Pores from mitochondrial outer membranes of yeast and a porin-deficient yeast mutant: a comparison. J Bioenerg Biomembr. 1989 Aug;21(4):439–450. doi: 10.1007/BF00762516. [DOI] [PubMed] [Google Scholar]
- Chich J. F., Goldschmidt D., Thieffry M., Henry J. P. A peptide-sensitive channel of large conductance is localized on mitochondrial outer membrane. Eur J Biochem. 1991 Feb 26;196(1):29–35. doi: 10.1111/j.1432-1033.1991.tb15781.x. [DOI] [PubMed] [Google Scholar]
- Coronado R., Latorre R. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys J. 1983 Aug;43(2):231–236. doi: 10.1016/S0006-3495(83)84343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Keller B. U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987 Nov 16;224(1):172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- Dihanich M., Schmid A., Oppliger W., Benz R. Identification of a new pore in the mitochondrial outer membrane of a porin-deficient yeast mutant. Eur J Biochem. 1989 May 15;181(3):703–708. doi: 10.1111/j.1432-1033.1989.tb14780.x. [DOI] [PubMed] [Google Scholar]
- Fèvre F., Chich J. F., Lauquin G. J., Henry J. P., Thieffry M. Comparison of mitochondrial cationic channels in wild-type and porin-deficient mutant yeast. FEBS Lett. 1990 Mar 26;262(2):201–204. doi: 10.1016/0014-5793(90)80189-p. [DOI] [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. P., Chich J. F., Goldschmidt D., Thieffry M. Blockade of a mitochondrial cationic channel by an addressing peptide: an electrophysiological study. J Membr Biol. 1989 Dec;112(2):139–147. doi: 10.1007/BF01871275. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I., Nagase H., Kishi K., Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991 Jul 18;352(6332):244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Campo M. L., Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr. 1989 Aug;21(4):497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Mitochondrial outer membrane channel (VDAC, porin) two-dimensional crystals from Neurospora. Methods Enzymol. 1986;125:595–610. doi: 10.1016/s0076-6879(86)25048-x. [DOI] [PubMed] [Google Scholar]
- Moran O., Sandri G., Panfili E., Stühmer W., Sorgato M. C. Electrophysiological characterization of contact sites in brain mitochondria. J Biol Chem. 1990 Jan 15;265(2):908–913. [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Moran O., De Pinto V., Keller B. U., Stuehmer W. Further investigation on the high-conductance ion channel of the inner membrane of mitochondria. J Bioenerg Biomembr. 1989 Aug;21(4):485–496. doi: 10.1007/BF00762520. [DOI] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Wan K., Lindstrom J., Montal M. Single-channel recordings from purified acetylcholine receptors reconstituted in bilayers formed at the tip of patch pipets. Biochemistry. 1983 May 10;22(10):2319–2323. doi: 10.1021/bi00279a003. [DOI] [PubMed] [Google Scholar]
- Tedeschi H., Mannella C. A., Bowman C. L. Patch clamping the outer mitochondrial membrane. J Membr Biol. 1987;97(1):21–29. doi: 10.1007/BF01869611. [DOI] [PubMed] [Google Scholar]
- Thieffry M., Chich J. F., Goldschmidt D., Henry J. P. Incorporation in lipid bilayers of a large conductance cationic channel from mitochondrial membranes. EMBO J. 1988 May;7(5):1449–1454. doi: 10.1002/j.1460-2075.1988.tb02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder U. R., Colombini M. Patch clamping VDAC in liposomes containing whole mitochondrial membranes. J Membr Biol. 1991 Jul;123(1):83–91. doi: 10.1007/BF01993966. [DOI] [PubMed] [Google Scholar]


