Abstract
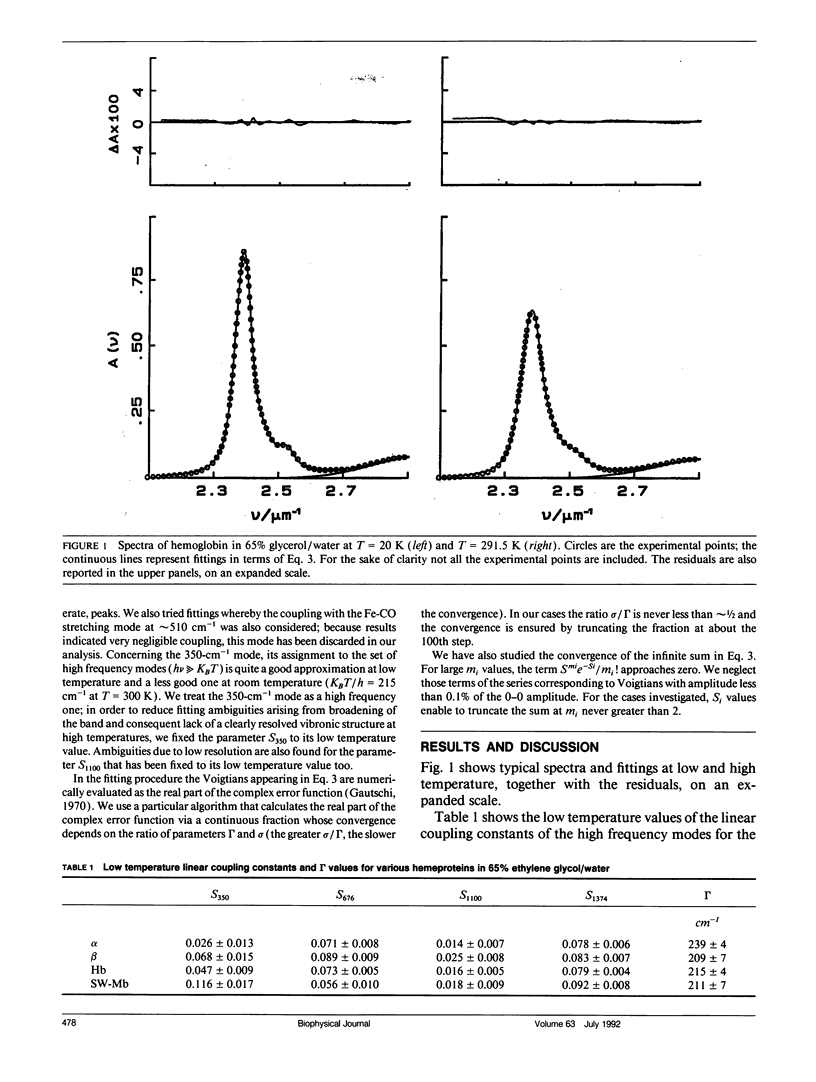
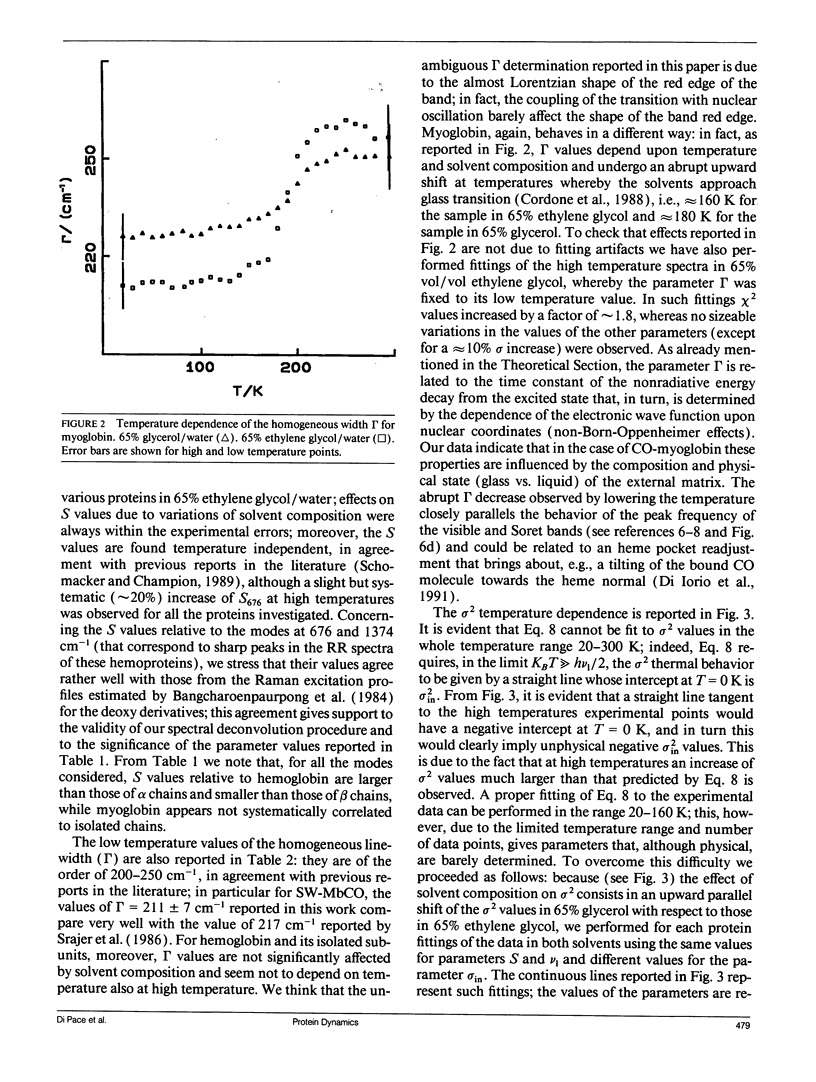
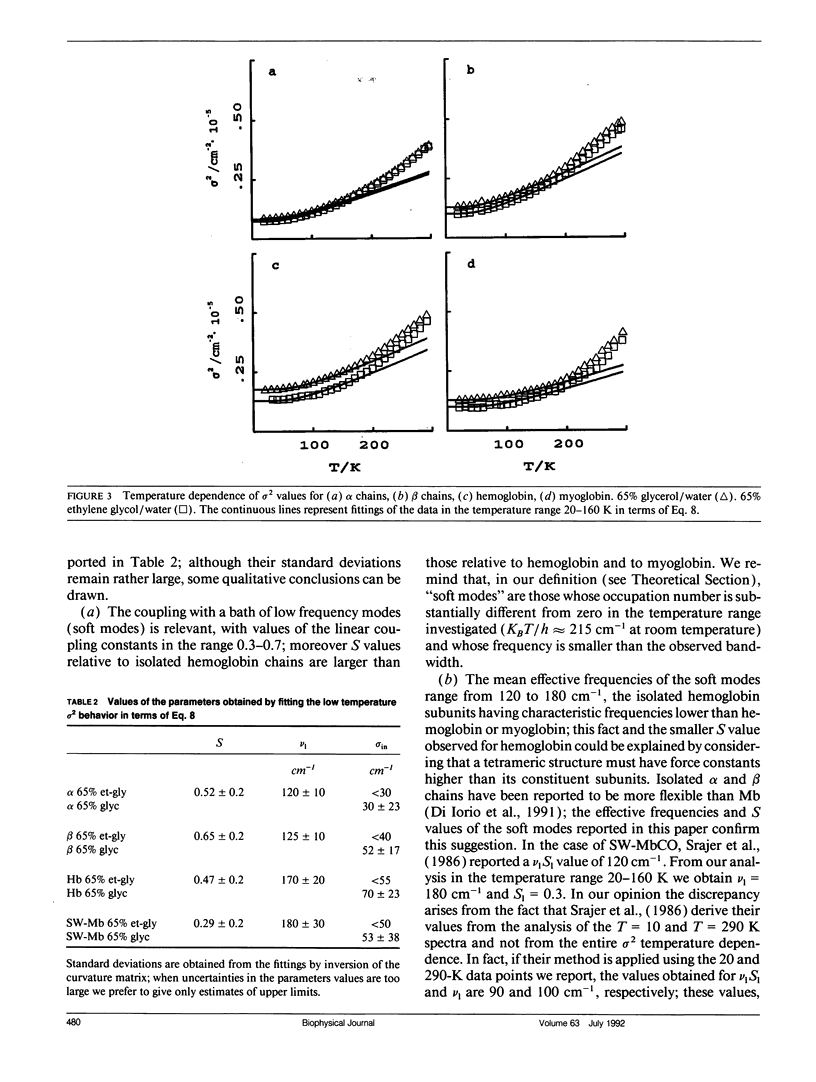
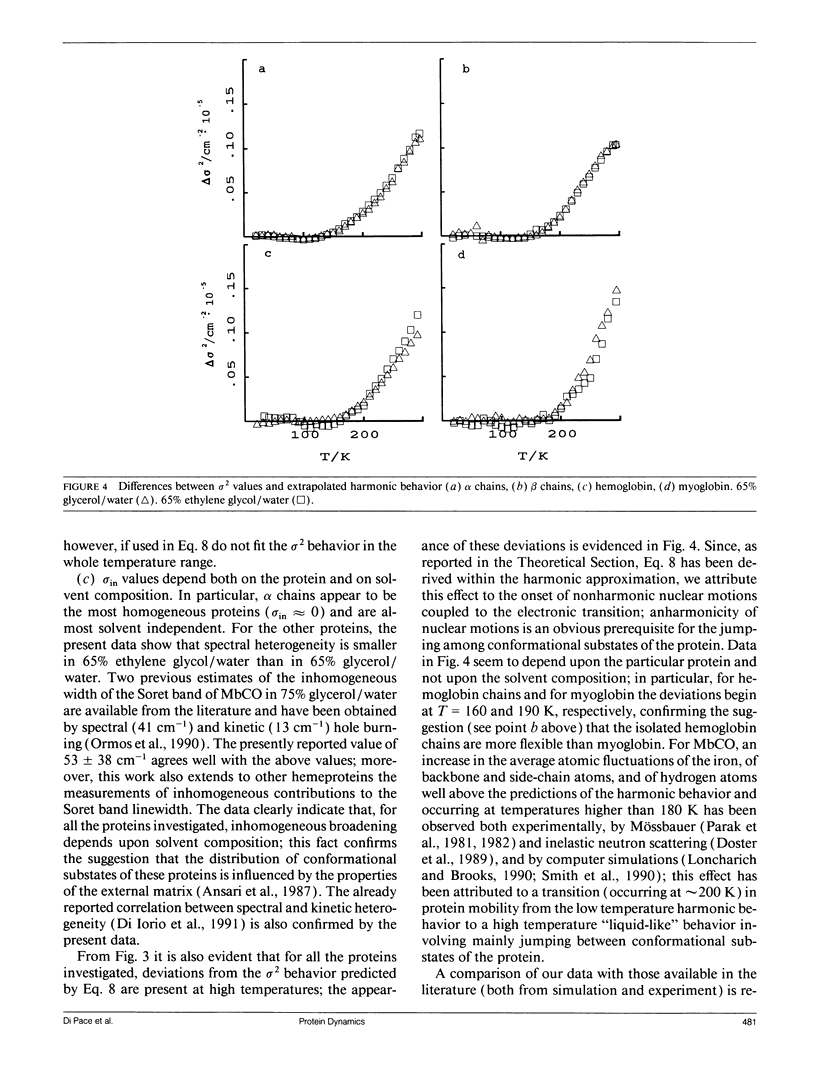
In this work we study the temperature dependence of the Soret band lineshape of the carbonmonoxy derivatives of sperm whale myoglobin, human hemoglobin, and its isolated alpha and beta subunits. To fit the observed spectral profile we use an analytic expression derived for a system whereby a single electronic transition is coupled to Franck-Condon active vibrational modes, within the adiabatic and harmonic approximation. The vibronic structure of the spectra arises from the coupling with high frequency modes; these modes contribute to the total line shape through a series of Lorentzians with peak positions at vibrational overtones and half width related to the time constant of the population decay of the excited electronic state (homogeneous broadening); moreover, the coupling with low frequency modes broadens each Lorentzian to a Voigtian. Inhomogeneous broadening is modeled as a gaussian distribution of the 0-0 transition frequencies and is therefore added as a constant term to the previous gaussian width. This spectral deconvolution enables us to investigate the different contributions to line broadening and the parameters that characterize the vibrational coupling, as well as their dependence upon protein and solvent composition. The investigation is carried out as a function of temperature in the range 20-300 K; relevant information is obtained by comparing experimental results with theoretical predictions. This work supports a description of the investigated proteins as heterogeneous systems, whose heterogeneity depends on the particular protein and on the composition of the external matrix. The delocalized pi electron cloud of the porphyrin ring is coupled not only to the high frequency vibrational modes of the active site but also to a "bath" of lower frequency modes that involve the entire protein; moreover at suitable temperatures (approximately 200 K), anharmonic motions, which are an obvious prerequisite for the jumping among different conformational substates, become evident.
Full text
PDF


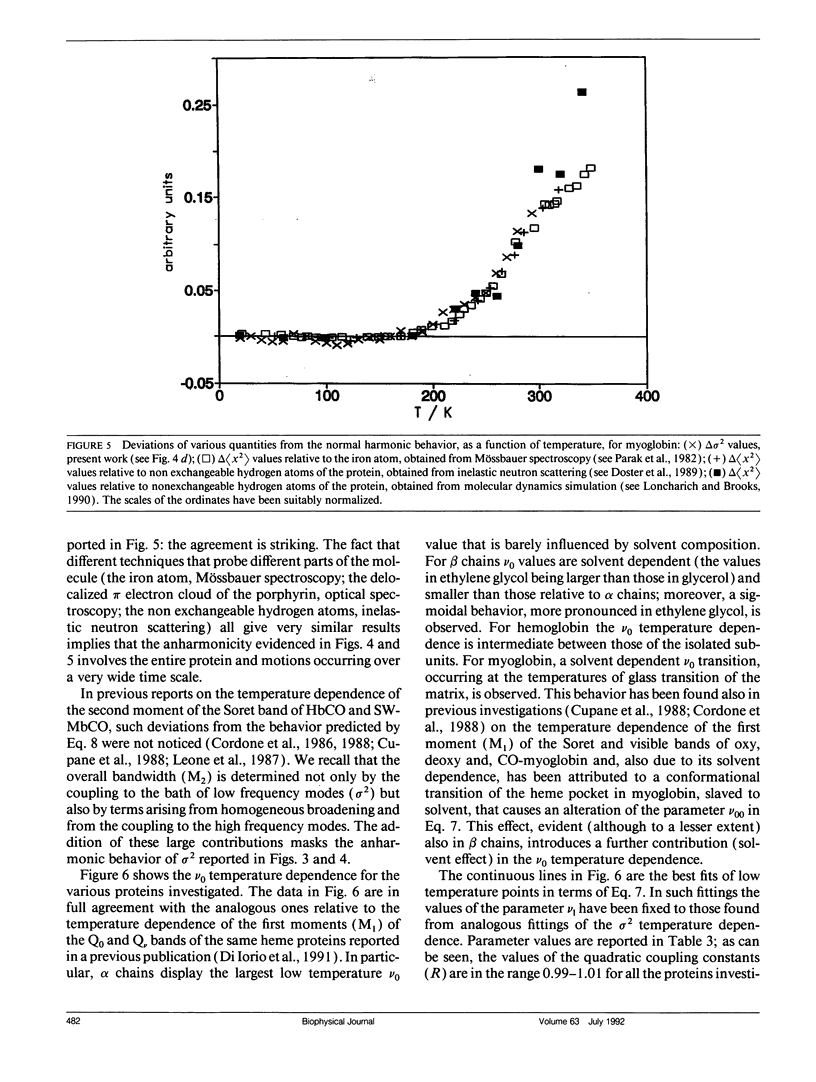
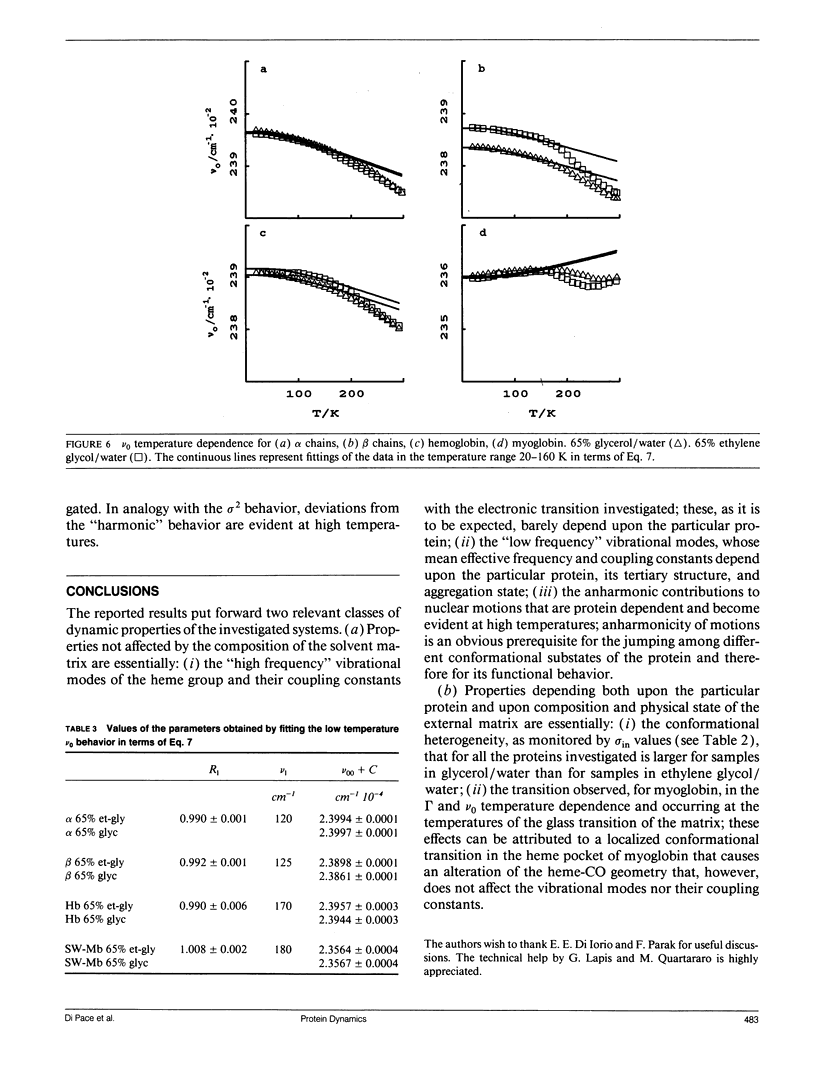

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Asher S. Resonance Raman spectroscopy of hemoglobin. Methods Enzymol. 1981;76:371–413. doi: 10.1016/0076-6879(81)76132-9. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E., Bulone D. Interaction between external medium and haem pocket in myoglobin probed by low-temperature optical spectroscopy. J Mol Biol. 1988 Jan 5;199(1):213–218. doi: 10.1016/0022-2836(88)90390-7. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys Chem. 1986 Aug;24(3):259–275. doi: 10.1016/0301-4622(86)85031-1. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., San Biagio P. L., Vitrano E. Effect of some monohydric alcohols on the oxygen affinity of hemoglobin: relevance of solvent dielectric constant and hydrophobicity. Biopolymers. 1979 Aug;18(8):1975–1988. doi: 10.1002/bip.1979.360180811. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Optical absorption spectra of azurin and stellacyanin in glycerol/water and ethylene glycol/water solutions in the temperature range 290-20 K. Biophys Chem. 1990 Nov;38(3):213–224. doi: 10.1016/0301-4622(90)87003-4. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Structural and dynamic properties of the heme pocket in myoglobin probed by optical spectroscopy. Biopolymers. 1988 Dec;27(12):1977–1997. doi: 10.1002/bip.360271209. [DOI] [PubMed] [Google Scholar]
- Di Iorio E. E., Hiltpold U. R., Filipovic D., Winterhalter K. H., Gratton E., Vitrano E., Cupane A., Leone M., Cordone L. Protein dynamics. Comparative investigation on heme-proteins with different physiological roles. Biophys J. 1991 Mar;59(3):742–754. doi: 10.1016/S0006-3495(91)82287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E. E. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Leone M., Cupane A., Vitrano E., Cordone L. Dynamic properties of oxy- and carbonmonoxyhemoglobin probed by optical spectroscopy in the temperature range of 300-20 K. Biopolymers. 1987 Oct;26(10):1769–1779. doi: 10.1002/bip.360261009. [DOI] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. Temperature dependence of dynamics of hydrated myoglobin. Comparison of force field calculations with neutron scattering data. J Mol Biol. 1990 Oct 5;215(3):439–455. doi: 10.1016/s0022-2836(05)80363-8. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- Smith J., Kuczera K., Karplus M. Dynamics of myoglobin: comparison of simulation results with neutron scattering spectra. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1601–1605. doi: 10.1073/pnas.87.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy)hemoglobin and -myoglobin: detection of Fe-CO stretching and Fe-C-O bending vibrations and influence of the quaternary structure change. Biochemistry. 1982 Mar 16;21(6):1132–1140. doi: 10.1021/bi00535a004. [DOI] [PubMed] [Google Scholar]


