Abstract
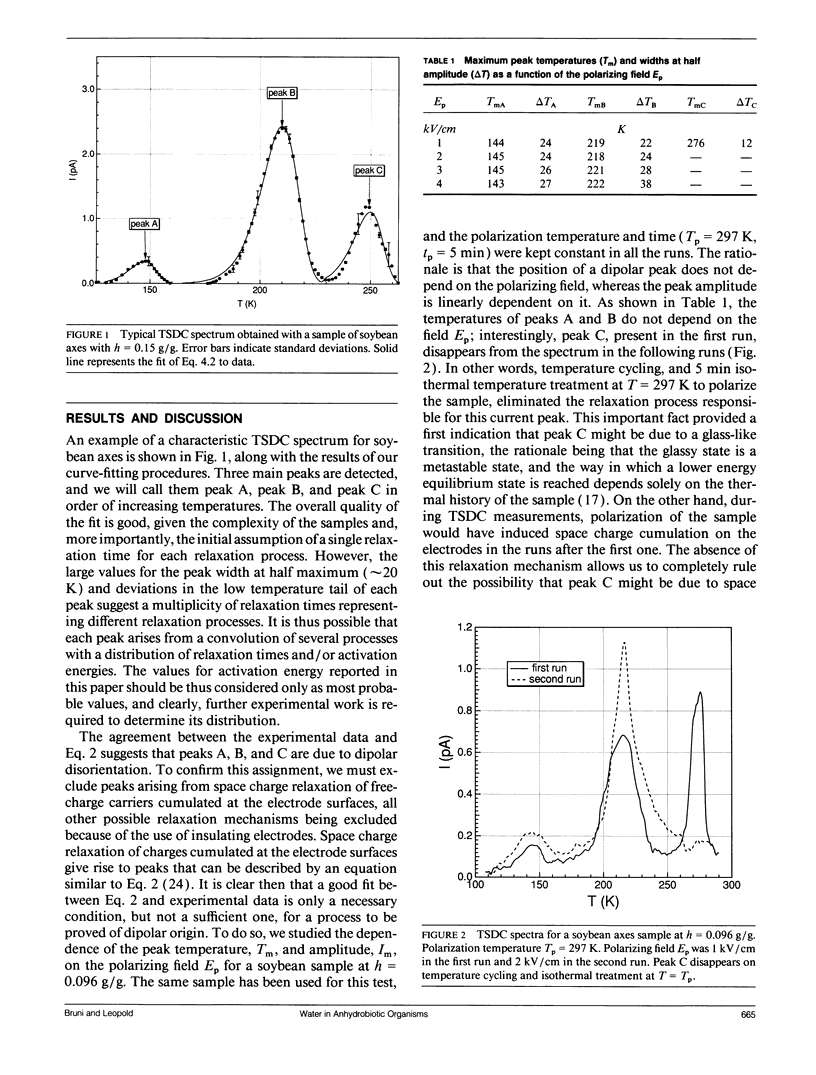
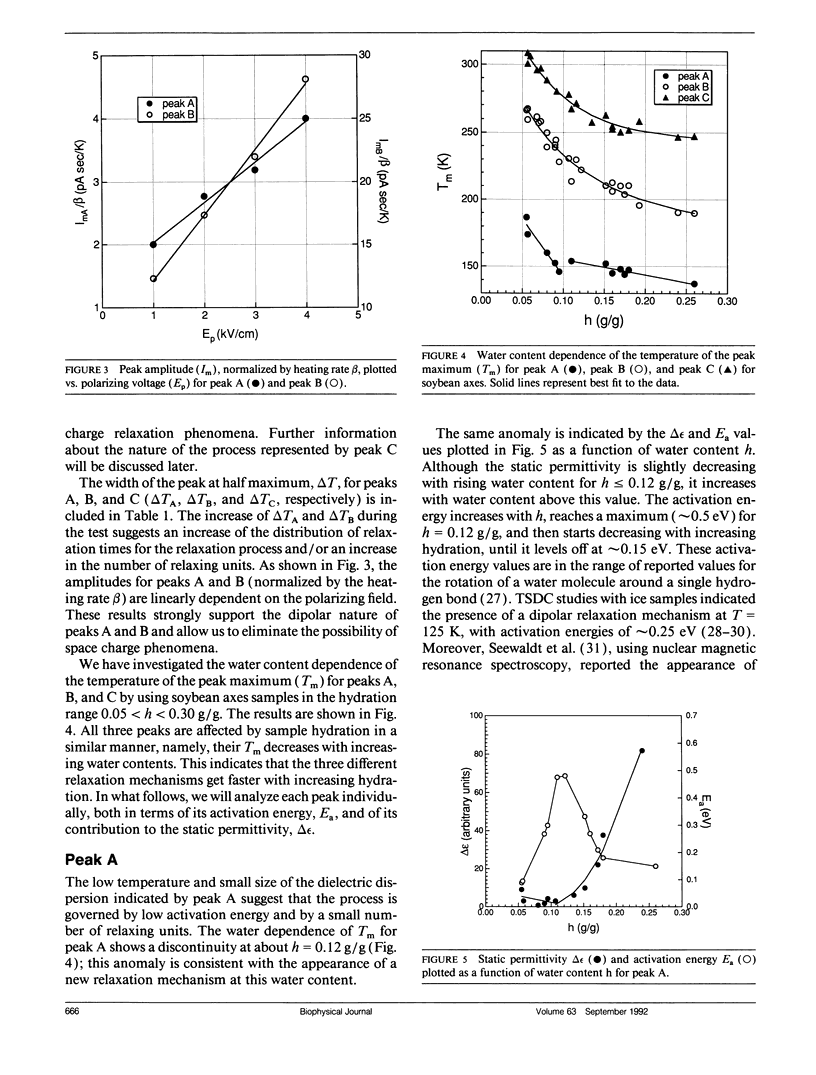
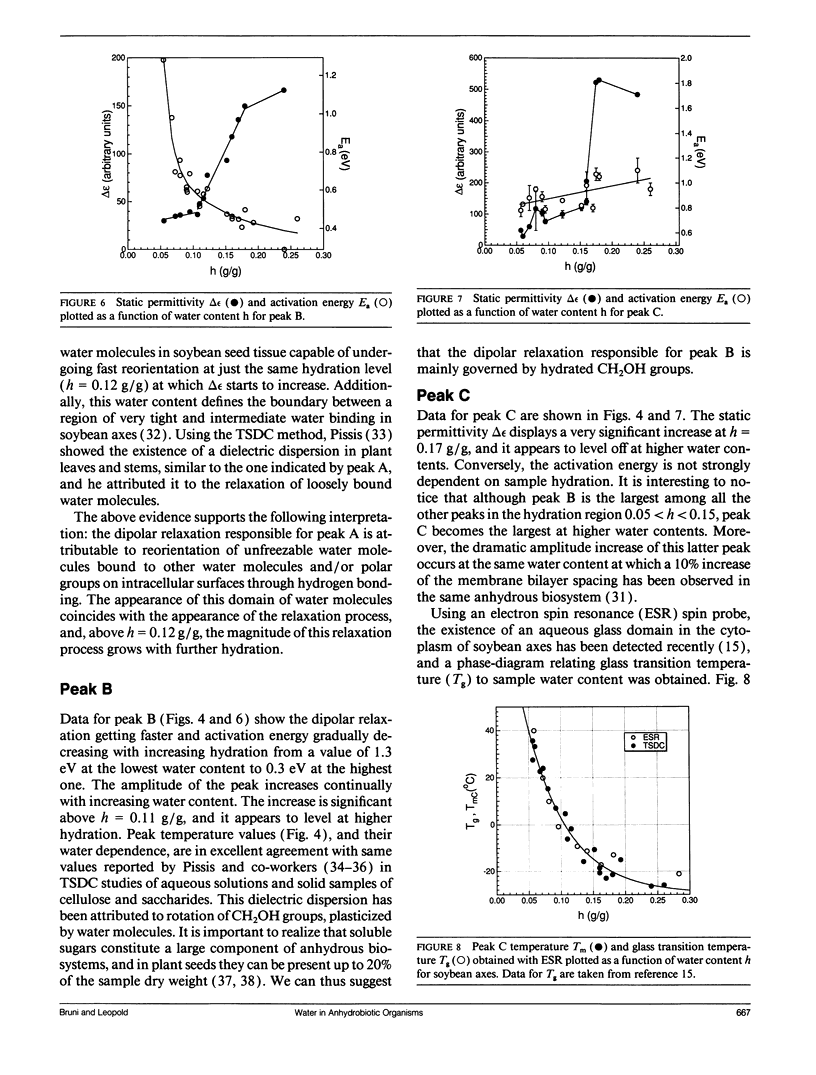
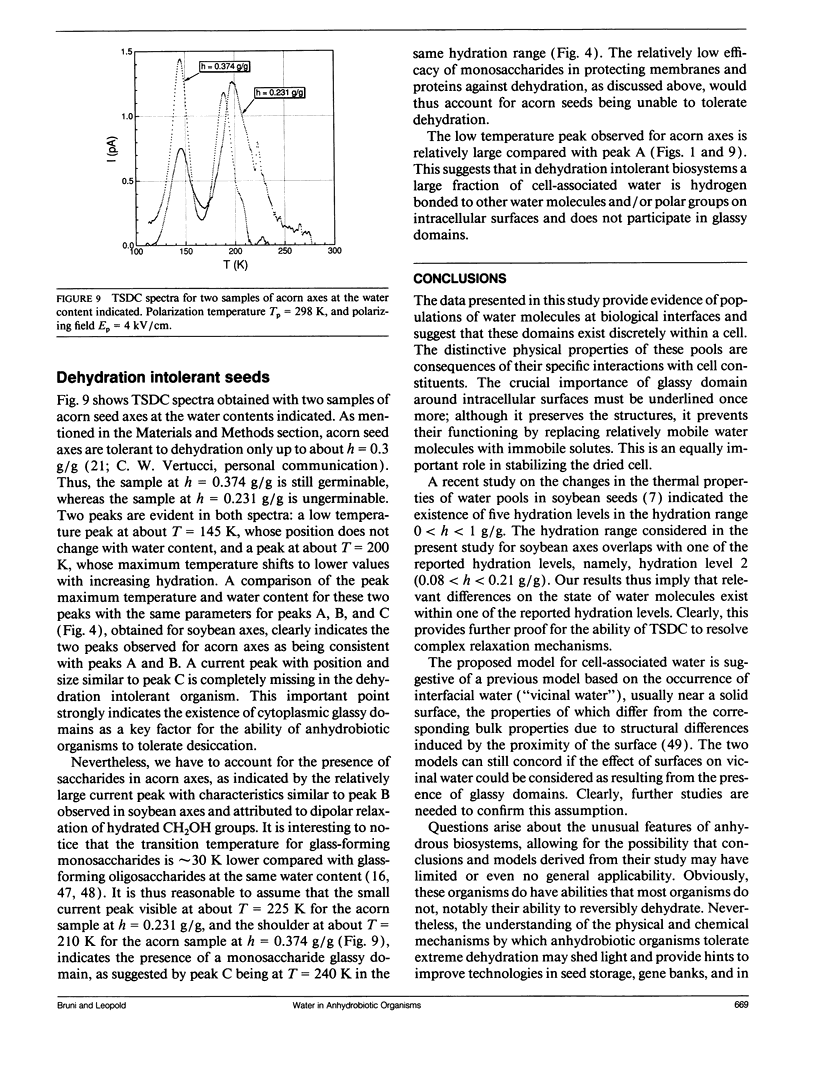
The ability to survive the removal of water in anhydrous biosystems is especially remarkable as a departure from the manifold structural and functional dependences on the presence of H2O molecules. Identifiable pools of water present in dry soybean axes were investigated by means of the thermally stimulated depolarization current method. Samples were examined in the temperature range 100-340 K and over water contents (h, in gram H2O per gram sample dry weight) ranging from h = 0.05 to 0.30 g/g. Three water-dependent relaxation mechanisms were detected; one attributed to dipolar reorientation of H2O molecules hydrogen-bonded to other water molecules, one to reorientation of CH2OH groups, and one to a glass transition in sugar-water domains. These glassy domains can protect intracellular components against destruction in the dehydrated state. Interestingly, protecting glassy domains were not found in dehydration intolerant seeds, supporting the hypothesis that the ability to withstand dehydration is associated with intracellular glass formation. A model for the state of cell water at interfaces is proposed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni F., Careri G., Clegg J. S. Dielectric properties of Artemia cysts at low water contents. Evidence for a percolative transition. Biophys J. 1989 Feb;55(2):331–338. doi: 10.1016/S0006-3495(89)82809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni F., Leopold A. C. Glass transitions in soybean seed : relevance to anhydrous biology. Plant Physiol. 1991 Jun;96(2):660–663. doi: 10.1104/pp.96.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni F, Careri G, Leopold AC. Critical exponents of protonic percolation in maize seeds. Phys Rev A Gen Phys. 1989 Sep 1;40(5):2803–2805. doi: 10.1103/physreva.40.2803. [DOI] [PubMed] [Google Scholar]
- Caffrey M., Fonseca V., Leopold A. C. Lipid-sugar interactions : relevance to anhydrous biology. Plant Physiol. 1988 Mar;86(3):754–758. doi: 10.1104/pp.86.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987 Feb 15;242(1):1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Rudolph A. S., Wistrom C. A., Spargo B. J., Anchordoguy T. J. Interactions of sugars with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Mouradian R., Crowe J. H., Jackson S. A., Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta. 1984 Jan 11;769(1):141–150. doi: 10.1016/0005-2736(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Koster K. L. Glass formation and desiccation tolerance in seeds. Plant Physiol. 1991 May;96(1):302–304. doi: 10.1104/pp.96.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster K. L., Leopold A. C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988 Nov;88(3):829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A. P. Non-equilibrium freezing behaviour of aqueous systems. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):167–189. doi: 10.1098/rstb.1977.0036. [DOI] [PubMed] [Google Scholar]
- Pammenter N. W., Vertucci C. W., Berjak P. Homeohydrous (Recalcitrant) Seeds: Dehydration, the State of Water and Viability Characteristics in Landolphia kirkii. Plant Physiol. 1991 Aug;96(4):1093–1098. doi: 10.1104/pp.96.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupley J. A., Careri G. Protein hydration and function. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Hirsh A. Calorimetric studies of the state of water in deeply frozen human monocytes. Biophys J. 1985 Mar;47(3):373–380. doi: 10.1016/S0006-3495(85)83928-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W. Calorimetric studies of the state of water in seed tissues. Biophys J. 1990 Dec;58(6):1463–1471. doi: 10.1016/S0006-3495(90)82491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W. Effects of cooling rate on seeds exposed to liquid nitrogen temperatures. Plant Physiol. 1989 Aug;90(4):1478–1485. doi: 10.1104/pp.90.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiol. 1984 May;75(1):114–117. doi: 10.1104/pp.75.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. The relationship between water binding and desiccation tolerance in tissues. Plant Physiol. 1987;85:232–238. doi: 10.1104/pp.85.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Leopold A. C. The glassy state in corn embryos. Plant Physiol. 1989 Mar;89(3):977–981. doi: 10.1104/pp.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]


