Abstract
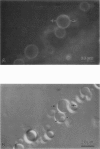
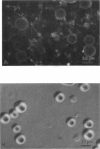
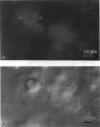
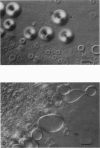
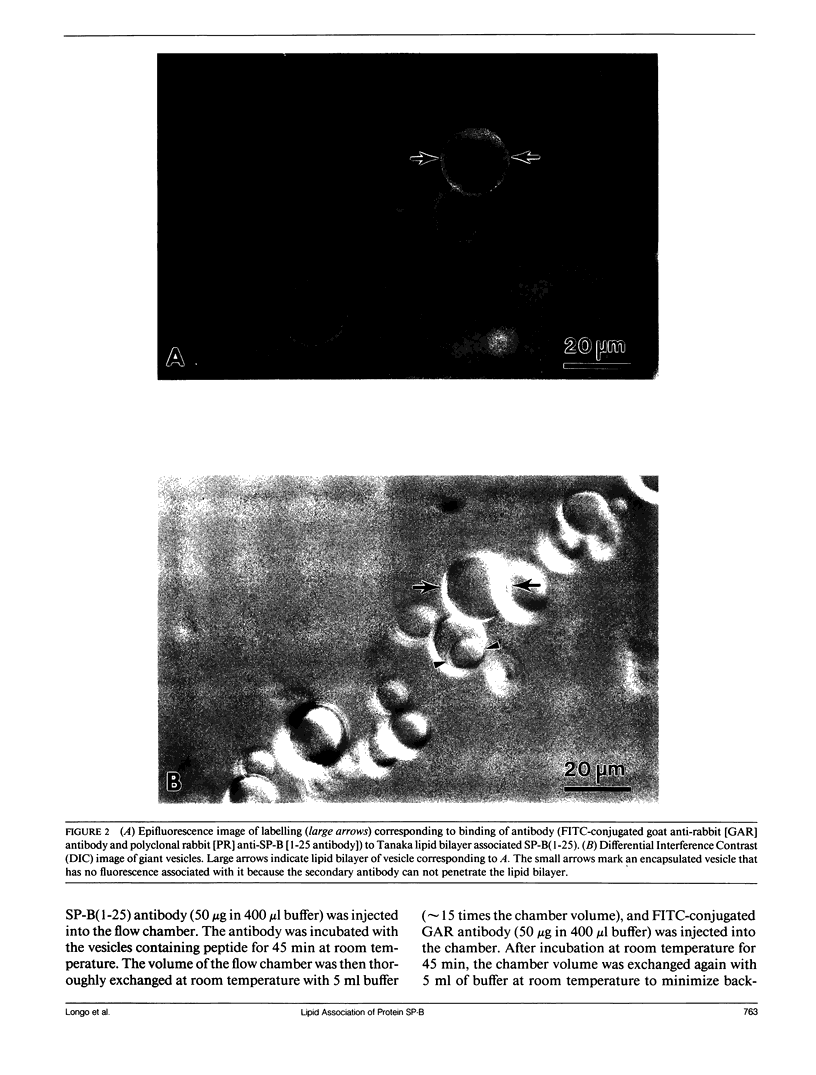
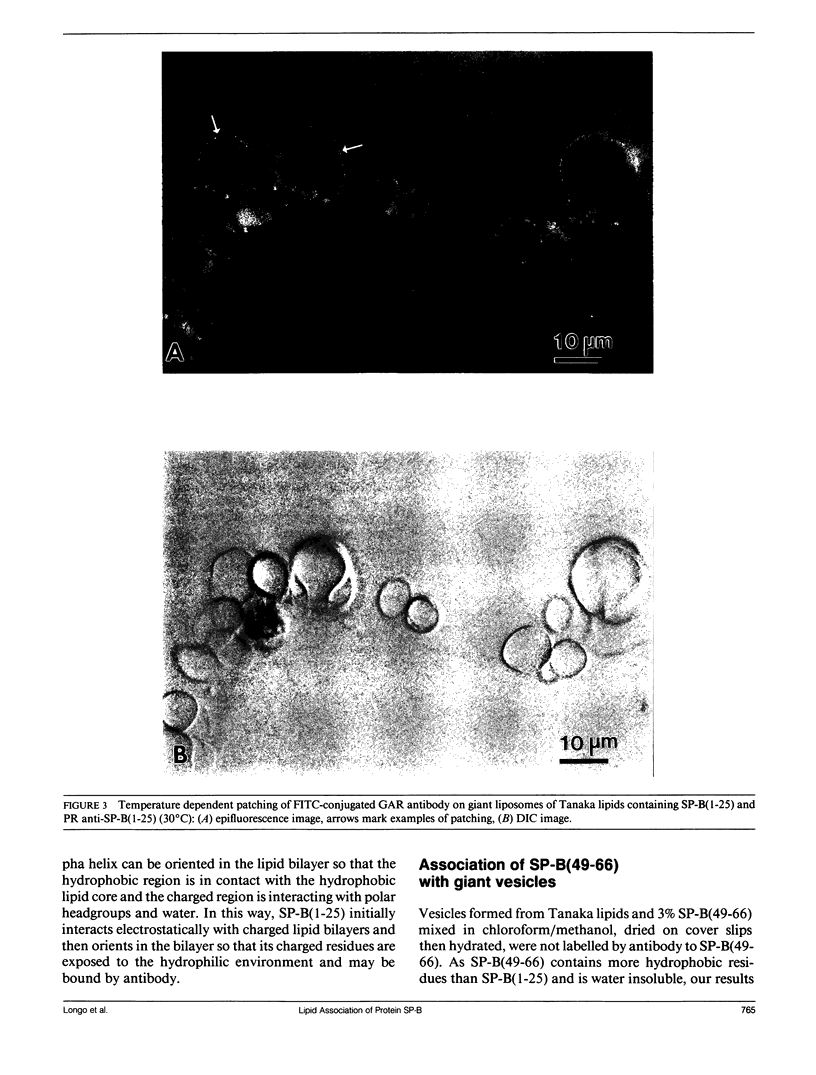
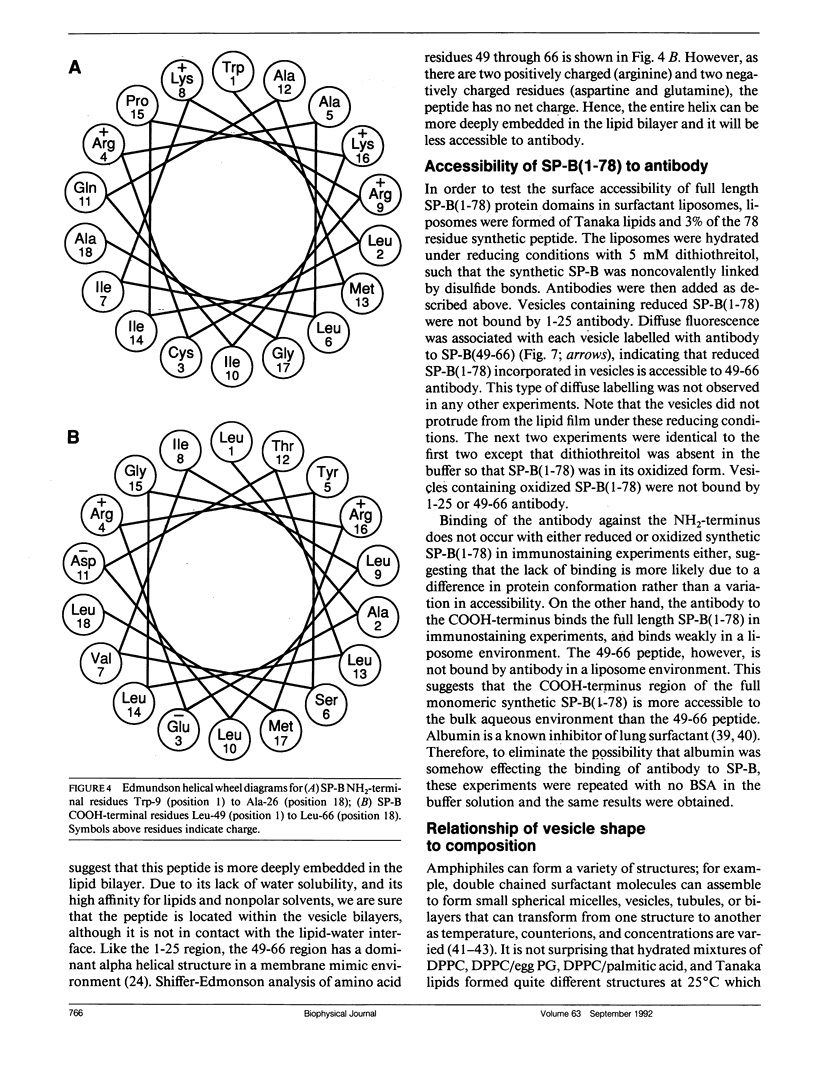
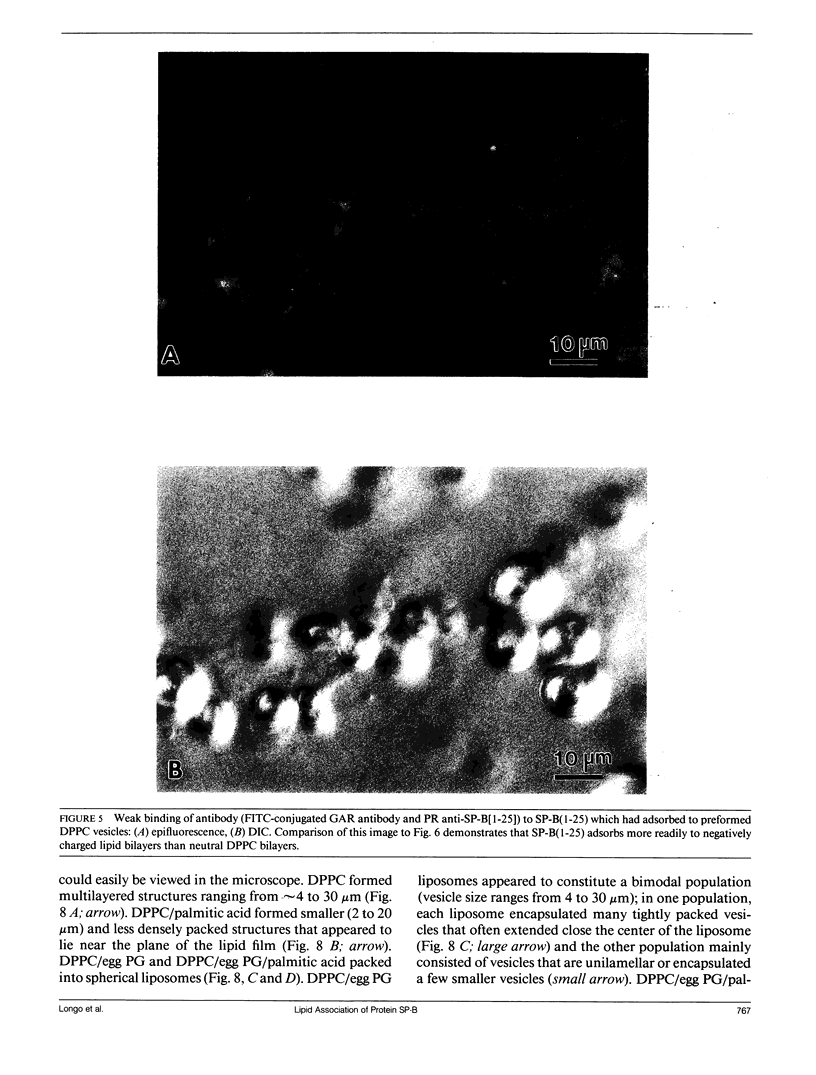
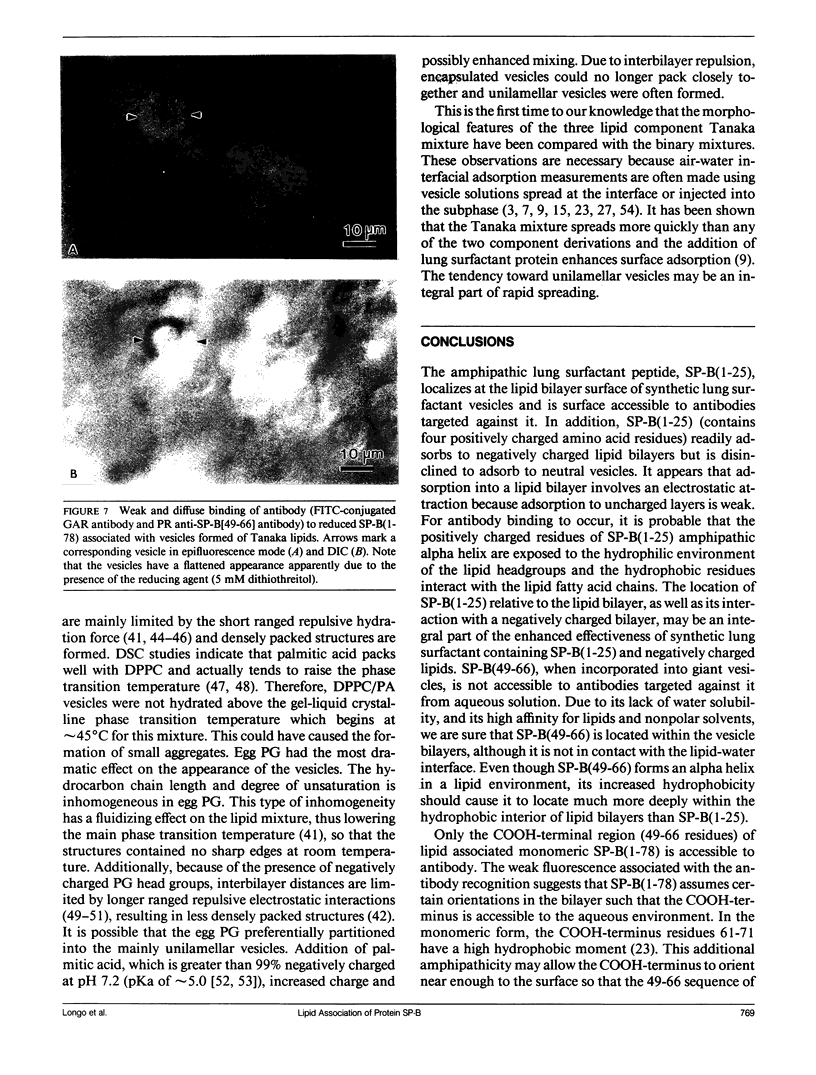
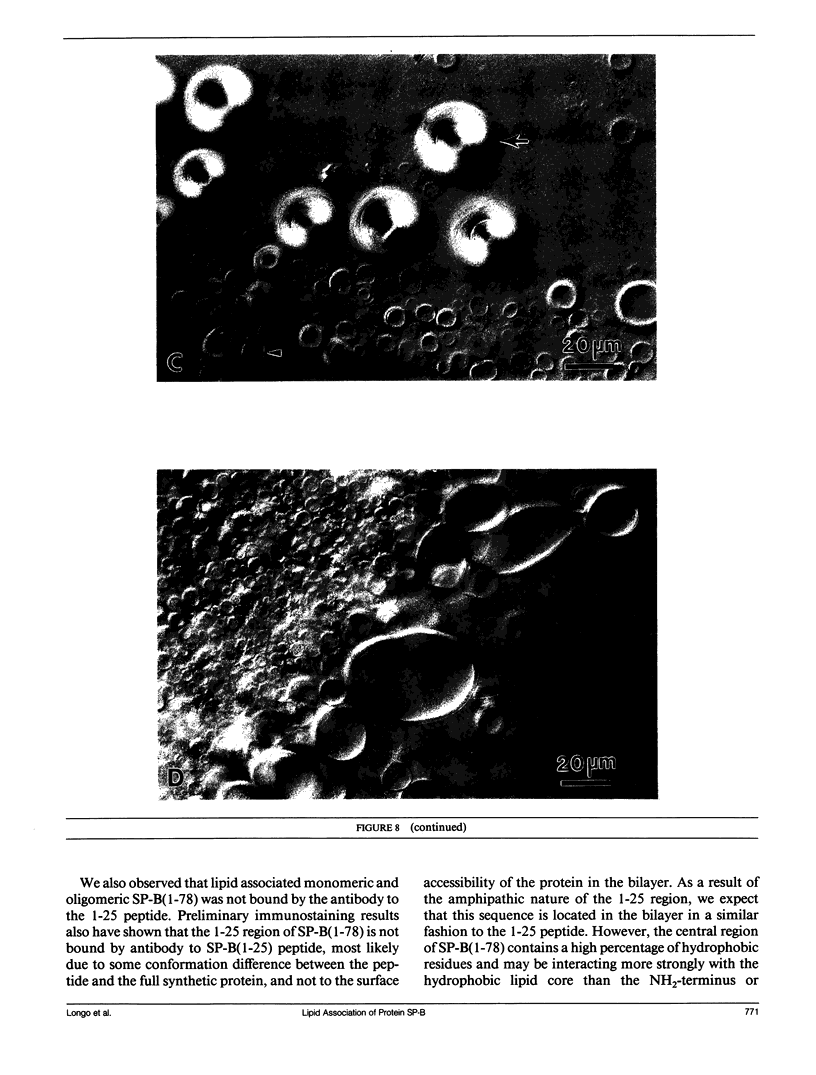
Lung surfactant protein, SP-B, and synthetic amphipathic peptides derived from SP-B were studied in model lung surfactant lipid bilayers by immunofluorescent labeling. Liposomes were formed by hydrating a lipid film on the glass viewing port of a temperature controlled flow chamber. Membrane associated peptides were detected by epifluorescence optical microscopy of the binding of anti-peptide polyclonal monospecific antibodies and FITC-conjugated secondary antibodies added to buffer contained in the flow chamber. Liposomes were bound by antibody to residues 1-25 of SP-B if formed from lipid films containing the 1-25 peptide, (SP-B(1-25)), or if SP-B(1-25) was added to already formed liposomes in buffer solution. The distribution of antigen-antibody complex was temperature dependent with aggregation occurring at greater than or equal to 30 degrees C. Surface association was not detected in liposomes formed from lipid films containing the 49-66 peptides (SP-B(49-66)), using an antibody to the 49-66 peptide, or to a synthetic version of the SP-B protein, (SP-B(1-78)), using both antibodies to the 49-66 peptide and the 1-25 peptide. The detection of SP-B(1-78) with antibody to the 49-66 sequence was only possible after reducing SP-B(1-78) with dithiothreitol, suggesting that the COOH-terminus of the full monomer protein is accessible to the bulk aqueous environment unlike the COOH-terminal peptide. The size, number of layers, and fluidity of the liposomes were not altered by protein or peptides, although they were affected by lipid composition and temperature.
Full text
PDF



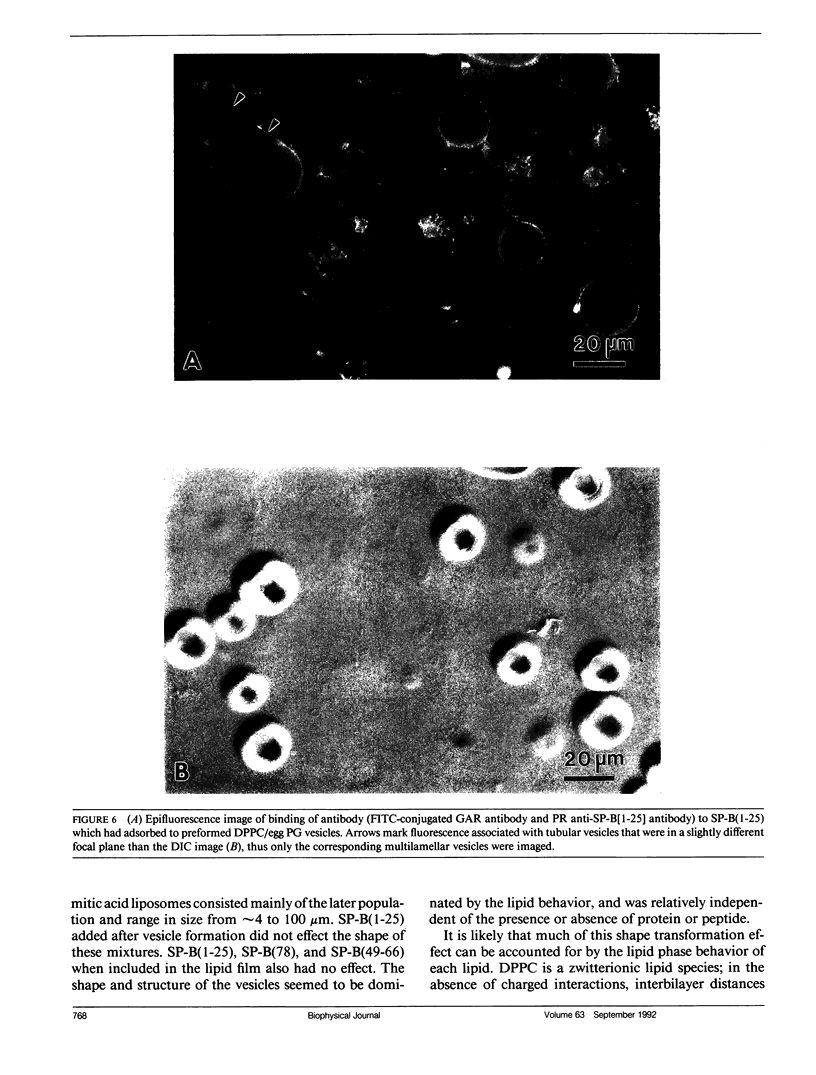



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Bruni R., Taeusch H. W., Waring A. J. Surfactant protein B: lipid interactions of synthetic peptides representing the amino-terminal amphipathic domain. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7451–7455. doi: 10.1073/pnas.88.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cockshutt A. M., Weitz J., Possmayer F. Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry. 1990 Sep 11;29(36):8424–8429. doi: 10.1021/bi00488a032. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Decher G., Ringsdorf H., Venzmer J., Bitter-Suermann D., Weisgerber C. Giant liposomes as model membranes for immunological studies: spontaneous insertion of purified K1-antigen (poly-alpha-2,8-NeuAc) of Escherichia coli. Biochim Biophys Acta. 1990 Apr 30;1023(3):357–364. doi: 10.1016/0005-2736(90)90127-a. [DOI] [PubMed] [Google Scholar]
- Egan E. A., Notter R. H., Kwong M. S., Shapiro D. L. Natural and artificial lung surfactant replacement therapy in premature lambs. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):875–883. doi: 10.1152/jappl.1983.55.3.875. [DOI] [PubMed] [Google Scholar]
- Fan B. R., Bruni R., Taeusch H. W., Findlay R., Waring A. J. Antibodies against synthetic amphipathic helical sequences of surfactant protein SP-B detect a conformational change in the native protein. FEBS Lett. 1991 May 6;282(2):220–224. doi: 10.1016/0014-5793(91)80481-h. [DOI] [PubMed] [Google Scholar]
- Fuchimukai T., Fujiwara T., Takahashi A., Enhorning G. Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol (1985) 1987 Feb;62(2):429–437. doi: 10.1152/jappl.1987.62.2.429. [DOI] [PubMed] [Google Scholar]
- GARVIN J. E., KARNOVSKY M. L. The titration of some phosphatides and related compounds in a non-aqueous medium. J Biol Chem. 1956 Jul;221(1):211–222. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T. E., Clark J. C., Pilot-Matias T., Meuth J., Fox J. L., Whitsett J. A. cDNA, deduced polypeptide structure and chromosomal assignment of human pulmonary surfactant proteolipid, SPL(pVal). J Biol Chem. 1988 Jan 5;263(1):9–12. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T., Pilot-Matias T., Fox J. L., Whitsett J. A. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci U S A. 1987 Jun;84(12):4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. D., Fieles W. E., Schotland D. L., Hogue-Angeletti R., Barchi R. L. Topographical localization of the C-terminal region of the voltage-dependent sodium channel from Electrophorus electricus using antibodies raised against a synthetic peptide. Proc Natl Acad Sci U S A. 1987 Jan;84(1):308–312. doi: 10.1073/pnas.84.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K. A., Phelps D. S., Steinbrink R., Fisch J., Kriz R., Mitsock L., Dougherty J. P., Taeusch H. W., Floros J. Isolation of a cDNA clone encoding a high molecular weight precursor to a 6-kDa pulmonary surfactant-associated protein. J Biol Chem. 1987 Jul 15;262(20):9808–9811. [PubMed] [Google Scholar]
- Jennings M. L. Topography of membrane proteins. Annu Rev Biochem. 1989;58:999–1027. doi: 10.1146/annurev.bi.58.070189.005031. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M. Surfactant for the treatment of respiratory distress syndrome. Am Rev Respir Dis. 1987 Nov;136(5):1256–1275. doi: 10.1164/ajrccm/136.5.1256. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Incorporation of saturated fatty acids into phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Mar 25;486(3):444–450. doi: 10.1016/0005-2760(77)90094-7. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Tilcock C. P., Wong K., Cullis P. R. Spontaneous vesiculation of large multilamellar vesicles composed of saturated phosphatidylcholine and phosphatidylglycerol mixtures. Biochemistry. 1988 Nov 29;27(24):8724–8730. doi: 10.1021/bi00424a006. [DOI] [PubMed] [Google Scholar]
- Marra J. Direct measurement of the interaction between phosphatidylglycerol bilayers in aqueous electrolyte solutions. Biophys J. 1986 Nov;50(5):815–825. doi: 10.1016/S0006-3495(86)83522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra J., Israelachvili J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry. 1985 Aug 13;24(17):4608–4618. doi: 10.1021/bi00338a020. [DOI] [PubMed] [Google Scholar]
- Mathialagan N., Possmayer F. Low-molecular-weight hydrophobic proteins from bovine pulmonary surfactant. Biochim Biophys Acta. 1990 Jul 16;1045(2):121–127. doi: 10.1016/0005-2760(90)90140-s. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem Phys Lipids. 1982 May;30(2-3):229–259. doi: 10.1016/0009-3084(82)90053-6. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Interactions between charged, uncharged, and zwitterionic bilayers containing phosphatidylglycerol. Biophys J. 1990 Jun;57(6):1187–1197. doi: 10.1016/S0006-3495(90)82638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Notter R. H., Shapiro D. L., Ohning B., Whitsett J. A. Biophysical activity of synthetic phospholipids combined with purified lung surfactant 6000 dalton apoprotein. Chem Phys Lipids. 1987 Jun;44(1):1–17. doi: 10.1016/0009-3084(87)90002-8. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Persson A., Rust K., Chang D., Moxley M., Longmore W., Crouch E. CP4: a pneumocyte-derived collagenous surfactant-associated protein. Evidence for heterogeneity of collagenous surfactant proteins. Biochemistry. 1988 Nov 15;27(23):8576–8584. doi: 10.1021/bi00423a011. [DOI] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Yu S. H., Weber J. M., Harding P. G. Pulmonary surfactant. Can J Biochem Cell Biol. 1984 Nov;62(11):1121–1133. doi: 10.1139/o84-146. [DOI] [PubMed] [Google Scholar]
- Seckler R., Möröy T., Wright J. K., Overath P. Anti-peptide antibodies and proteases as structural probes for the lactose/H+ transporter of Escherichia coli: a loop around amino acid residue 130 faces the cytoplasmic side of the membrane. Biochemistry. 1986 May 6;25(9):2403–2409. doi: 10.1021/bi00357a016. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Shiffer K., Hawgood S., Düzgünes N., Goerke J. Interactions of the low molecular weight group of surfactant-associated proteins (SP 5-18) with pulmonary surfactant lipids. Biochemistry. 1988 Apr 19;27(8):2689–2695. doi: 10.1021/bi00408a008. [DOI] [PubMed] [Google Scholar]
- Smith G. B., Taeusch H. W., Phelps D. S., Keough K. M. Mixtures of low molecular weight surfactant proteins and dipalmitoyl phosphatidylcholine duplicate effects of pulmonary surfactant in vitro and in vivo. Pediatr Res. 1988 May;23(5):484–490. doi: 10.1203/00006450-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Curstedt T., Grossmann G., Kobayashi T., Nilsson R., Nohara K., Robertson B. The role of the low-molecular weight (less than or equal to 15,000 daltons) apoproteins of pulmonary surfactant. Eur J Respir Dis. 1986 Nov;69(5):336–345. [PubMed] [Google Scholar]
- Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989 Jul;140(1):75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- Taeusch H. W., Keough K. M., Williams M., Slavin R., Steele E., Lee A. S., Phelps D., Kariel N., Floros J., Avery M. E. Characterization of bovine surfactant for infants with respiratory distress syndrome. Pediatrics. 1986 Apr;77(4):572–581. [PubMed] [Google Scholar]
- Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986 Mar 13;135(2):527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Waring A. J., Amirkhanian J., Fan B., Taeusch H. W. Structure-function relationships of bovine pulmonary surfactant proteins: SP-B and SP-C. Biochim Biophys Acta. 1990 May 1;1044(1):43–49. doi: 10.1016/0005-2760(90)90216-k. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Takei T., Aiba T., Masuda K., Kiuchi A., Fujiwara T. Development of synthetic lung surfactants. J Lipid Res. 1986 May;27(5):475–485. [PubMed] [Google Scholar]
- Tanaka Y., Takei T., Kanazawa Y. Lung surfactants. II. Effects of fatty acids, triacylglycerols and protein on the activity of lung surfactant. Chem Pharm Bull (Tokyo) 1983 Nov;31(11):4100–4109. doi: 10.1248/cpb.31.4100. [DOI] [PubMed] [Google Scholar]
- Waring A., Taeusch W., Bruni R., Amirkhanian J., Fan B., Stevens R., Young J. Synthetic amphipathic sequences of surfactant protein-B mimic several physicochemical and in vivo properties of native pulmonary surfactant proteins. Pept Res. 1989 Sep-Oct;2(5):308–313. [PubMed] [Google Scholar]
- Warr R. G., Hawgood S., Buckley D. I., Crisp T. M., Schilling J., Benson B. J., Ballard P. L., Clements J. A., White R. T. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]