Abstract
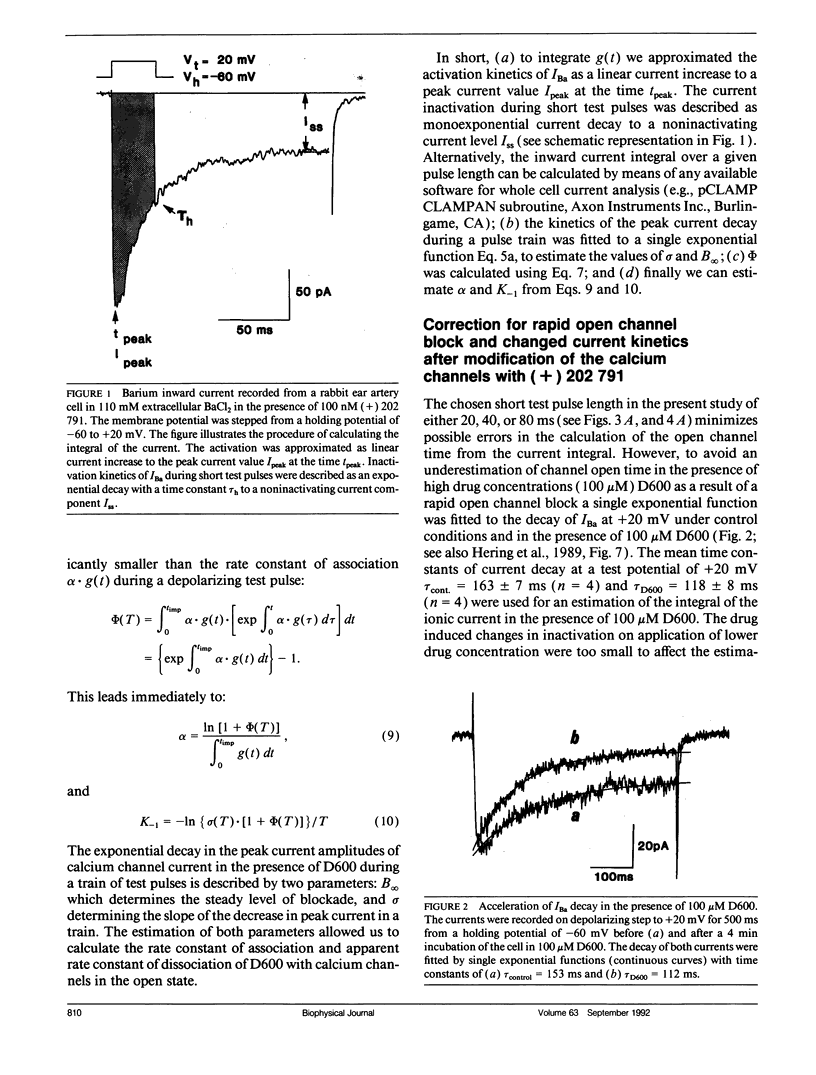
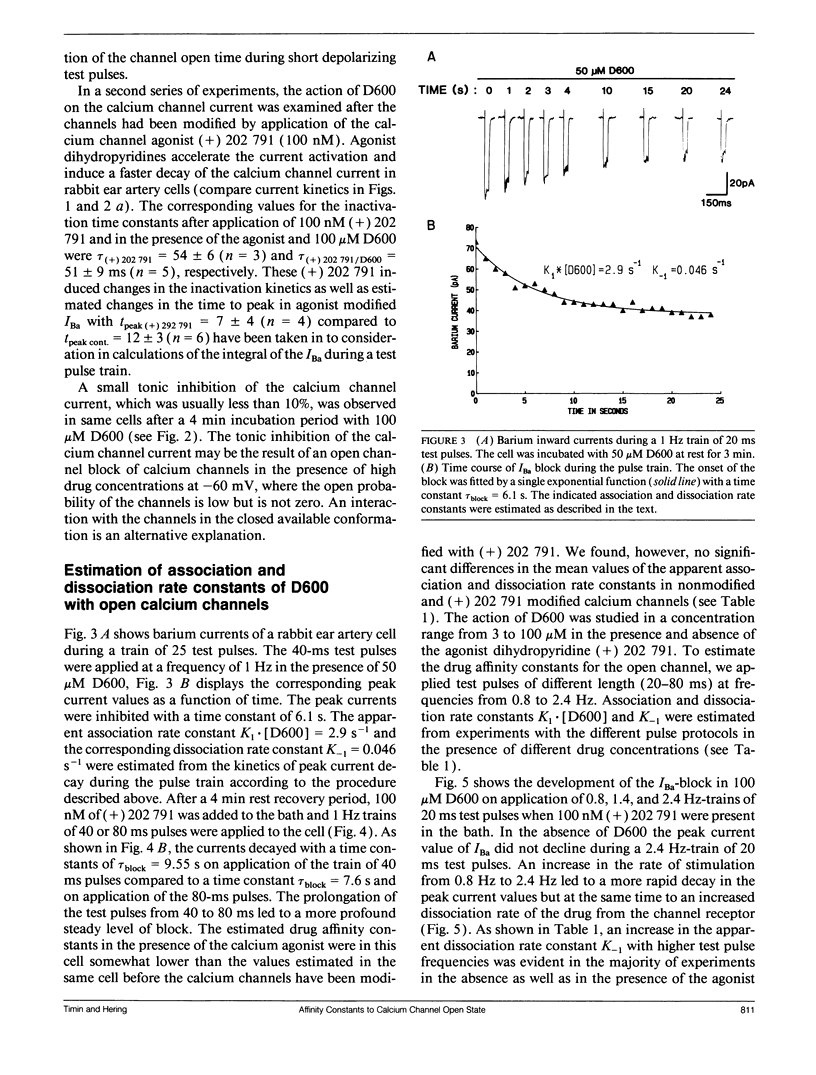
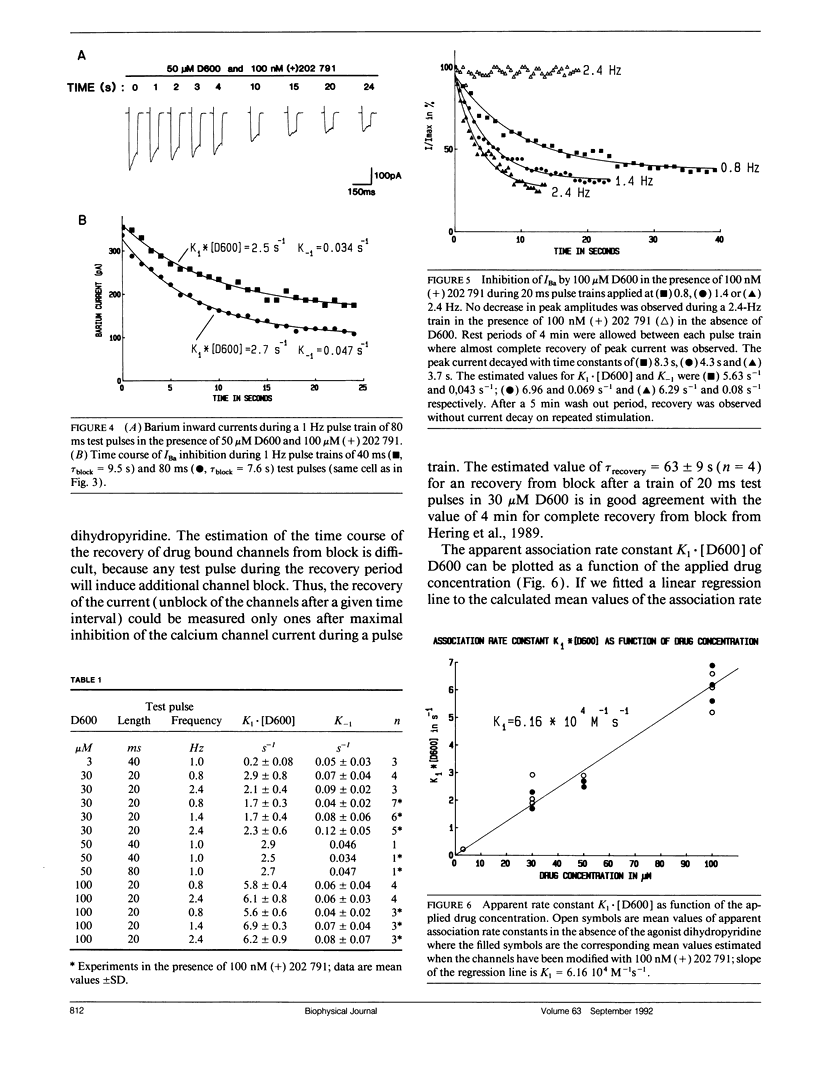
The affinity of D600 to calcium channels in the open state has been examined in isolated smooth muscle cells of the rabbit ear artery. Calcium channel currents were measured in high external barium solution by means of the patch-clamp technique. The current inhibition in various D600 concentrations (3-100 microM) on application of trains of short test pulses (20-80 ms) has been studied in nonmodified calcium channels and in cells where the calcium channels were modified by the agonist dihydropyridine (+) 202,791 (100 nM). The kinetics of the peak current decay has been analyzed with a mathematical model which is based on the experimental finding that D600 interacts primarily with calcium channels in the open conformational state. The model approach allows the estimation of drug affinity constants of D600 to the calcium channel in the open conformation. An association rate constant to the open conformational state of D600 of 6.16 x 10(4) M-1 s-1 was estimated. The association rate of the drug was not significantly changed after the calcium channels have been modified with 100 nM (+) 202,791. A method for correction of rate constants for possible drug trapping is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chernoff D. M. Kinetic analysis of phasic inhibition of neuronal sodium currents by lidocaine and bupivacaine. Biophys J. 1990 Jul;58(1):53–68. doi: 10.1016/S0006-3495(90)82353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R., Kendig J. J., Cohen E. N. The rates of interaction of local anesthetics with sodium channels in nerve. J Pharmacol Exp Ther. 1978 Nov;207(2):594–604. [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Calcium channels. Vitam Horm. 1988;44:155–328. doi: 10.1016/s0083-6729(08)60695-0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hering S., Bolton T. B., Beech D. J., Lim S. P. Mechanism of calcium channel block by D600 in single smooth muscle cells from rabbit ear artery. Circ Res. 1989 May;64(5):928–936. doi: 10.1161/01.res.64.5.928. [DOI] [PubMed] [Google Scholar]
- Hering S., Kleppisch T., Timin E. N., Bodewei R. Characterization of the calcium channel state transitions induced by the enantiomers of the 1,4-dihydropyridine Sandoz 202 791 in neonatal rat heart cells. A nonmodulated receptor model. Pflugers Arch. 1989 Sep;414(6):690–700. doi: 10.1007/BF00582137. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Tiapamil reduces the calcium inward current of isolated smooth muscle cells. Dependence on holding potential and pulse frequency. Eur J Pharmacol. 1986 Aug 15;127(3):165–171. doi: 10.1016/0014-2999(86)90360-2. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Haap K. The blockade of Vmax of the atrioventricular action potential produced by the slow channel inhibitors verapamil and nifedipine. Naunyn Schmiedebergs Arch Pharmacol. 1981 Apr;316(2):178–185. doi: 10.1007/BF00505314. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Brown A. M. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989 Jun;93(6):1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y., Hori N., Tokutomi N., Akaike N. D-600 blocks open Ca2+ channels more profoundly than closed ones. Brain Res. 1987 Aug 4;417(1):143–147. doi: 10.1016/0006-8993(87)90189-2. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O. Phasic ion channel blockade. A kinetic model and parameter estimation procedure. Mol Pharmacol. 1985 Oct;28(4):348–356. [PubMed] [Google Scholar]


