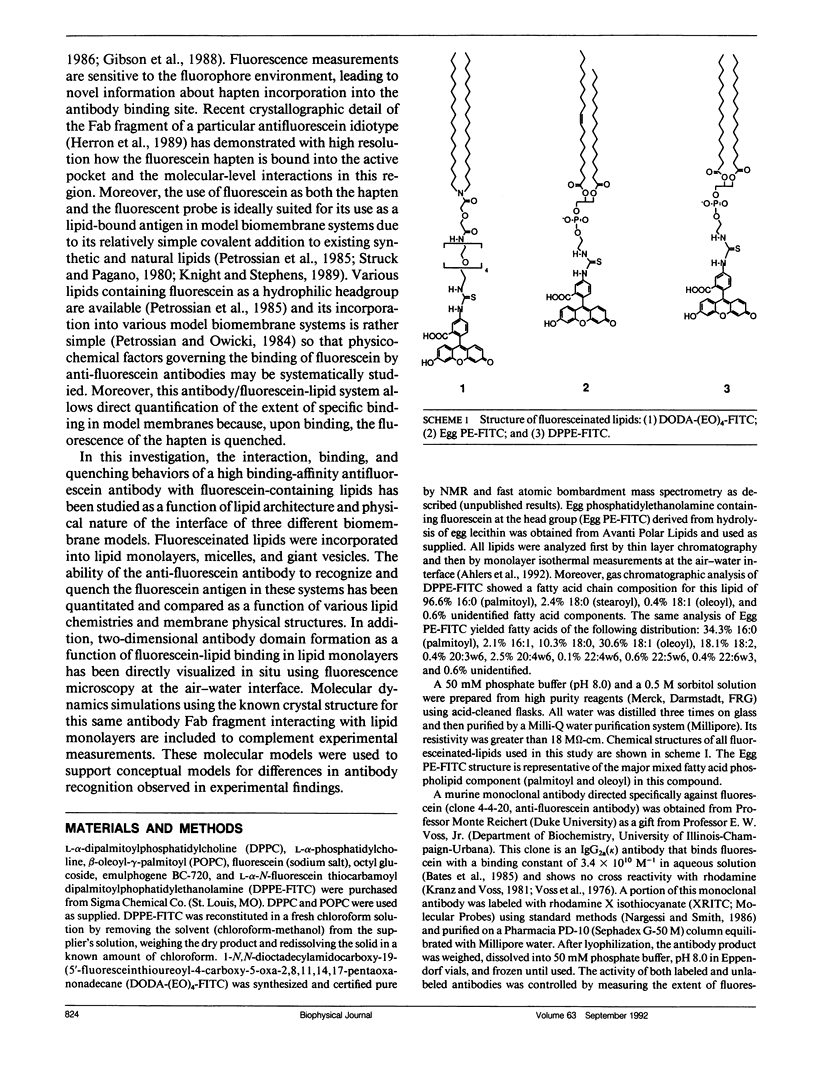
Abstract
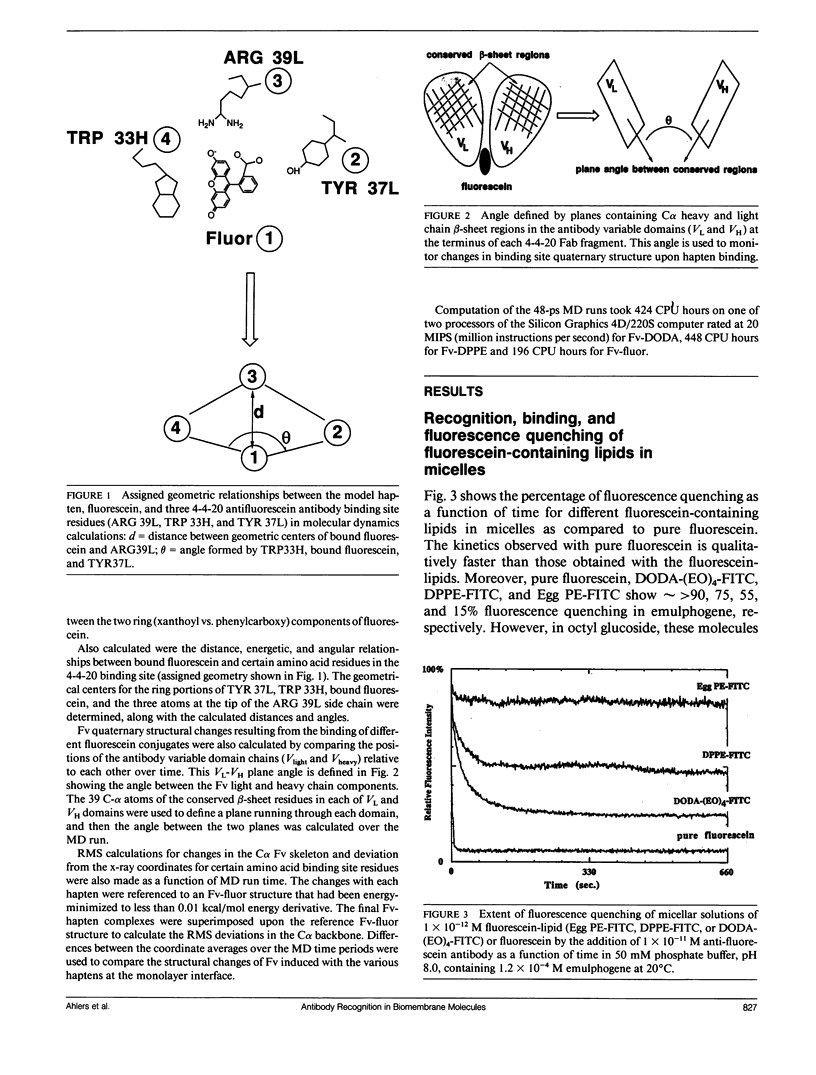
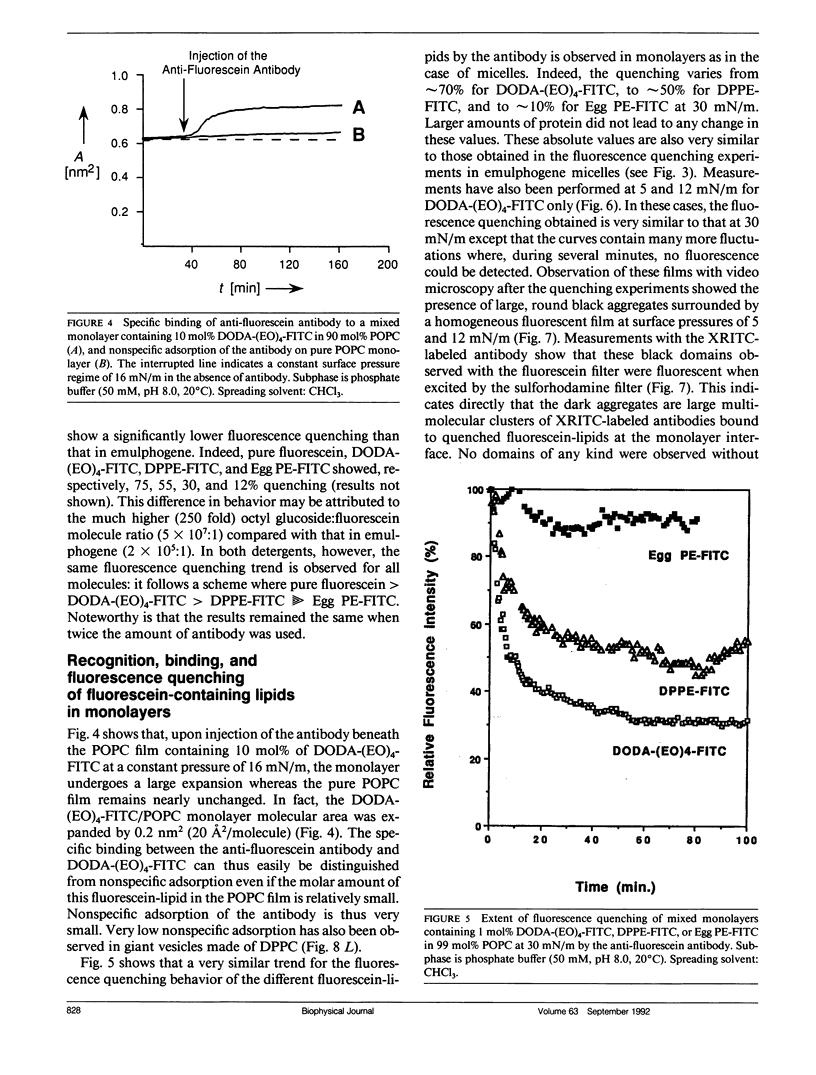
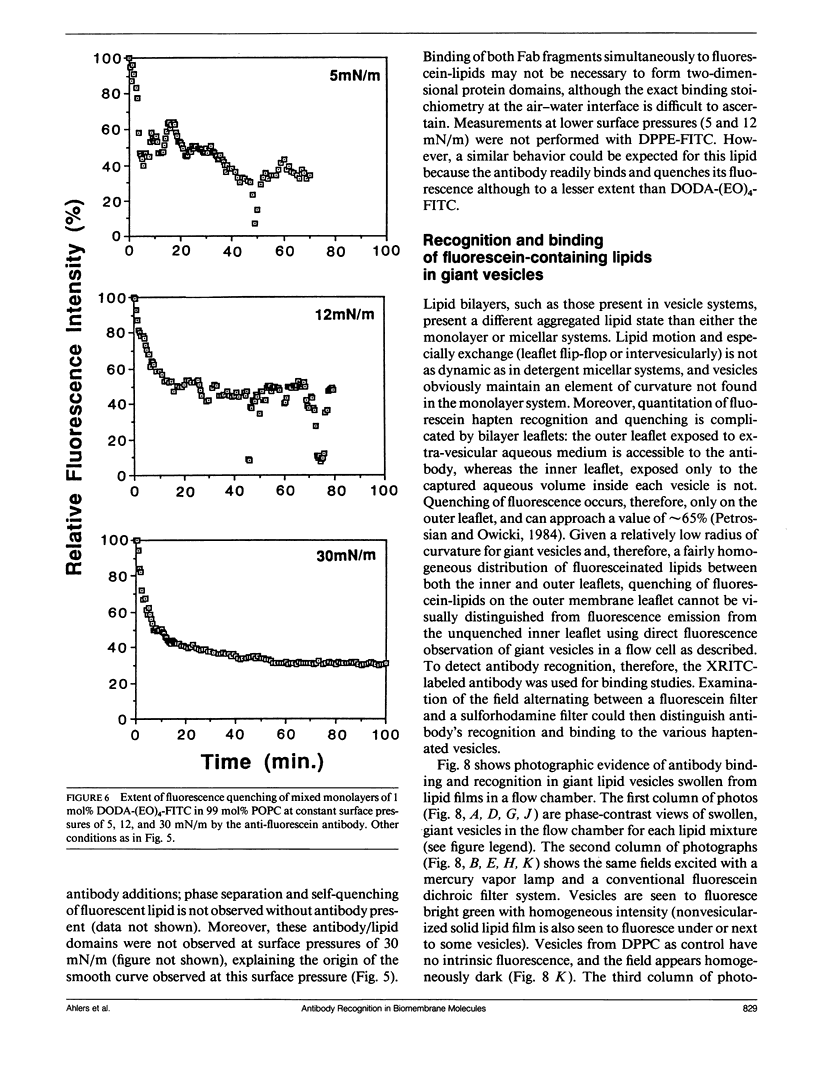
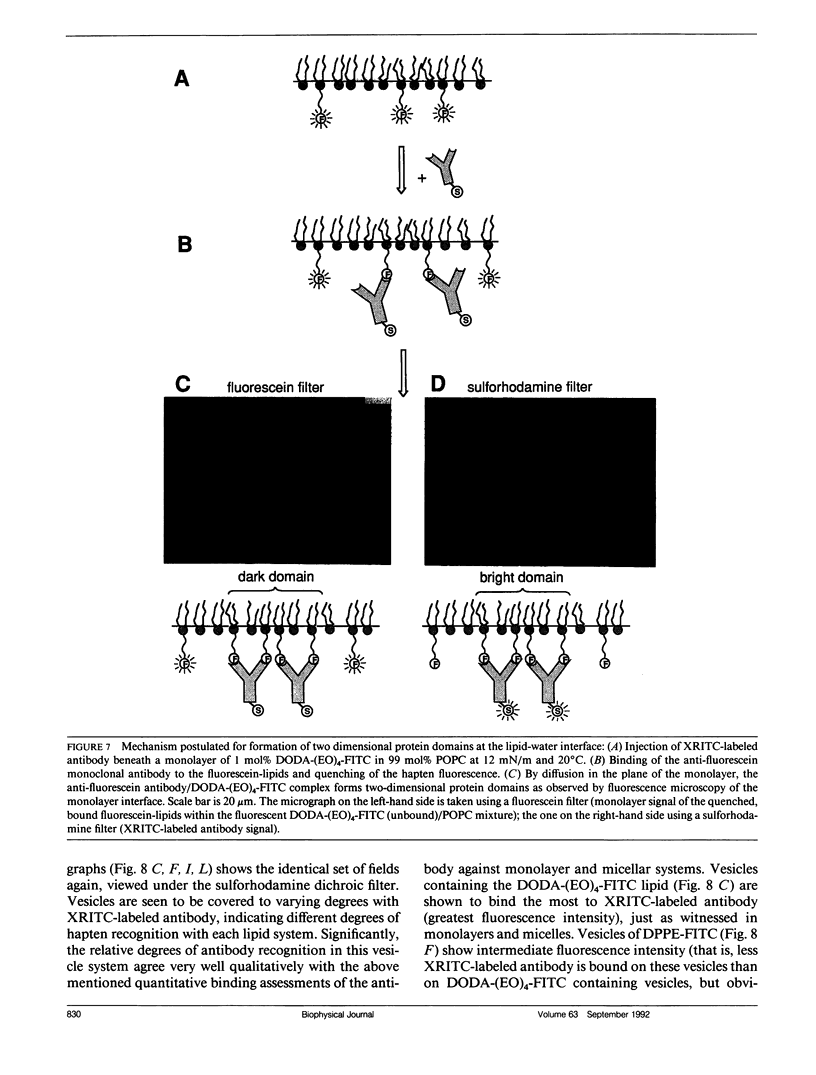
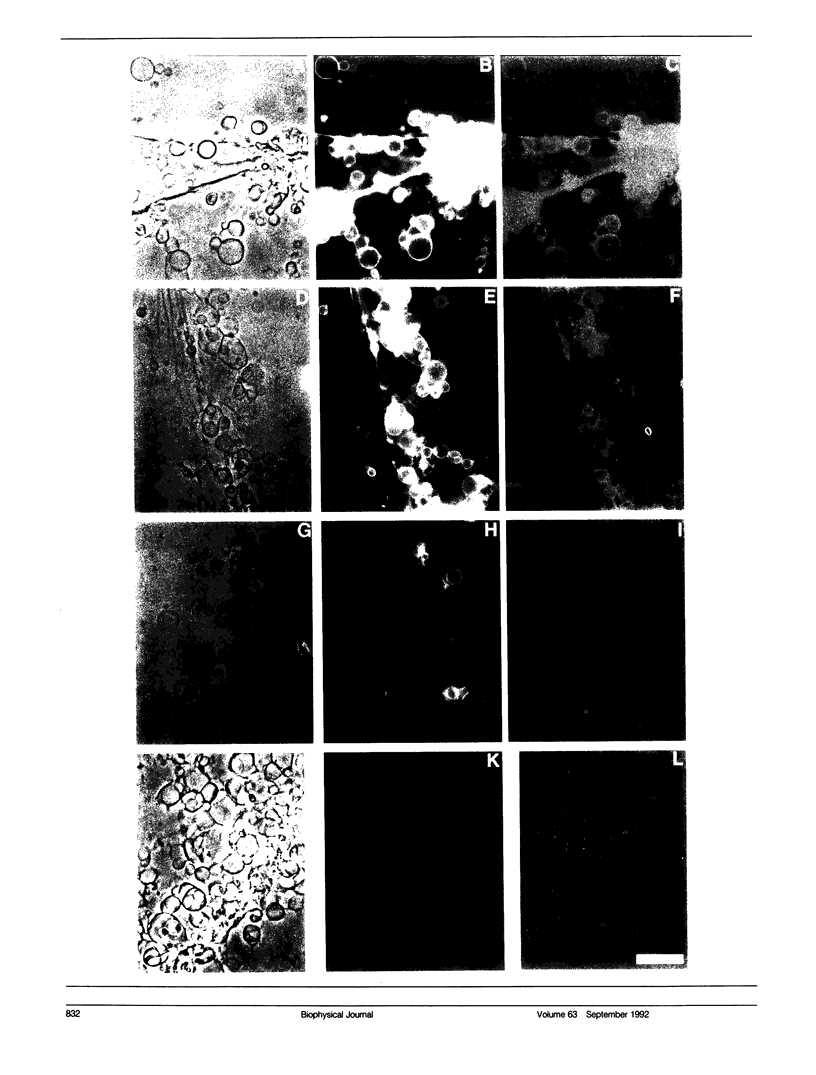
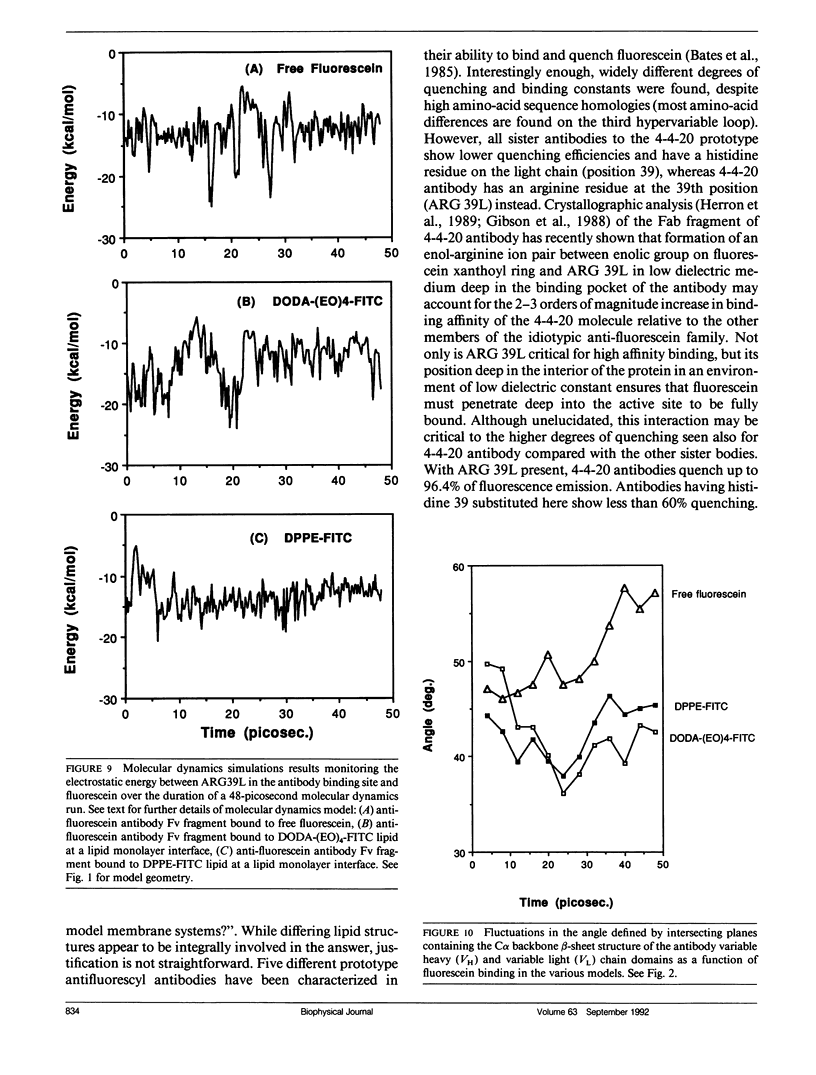
Three model biomembrane systems, monolayers, micelles, and vesicles, have been used to study the influence of chemical and physical variables of hapten presentation at membrane interfaces on antibody binding. Hapten recognition and binding were monitored for the anti-fluorescein monoclonal antibody 4-4-20 generated against the hapten, fluorescein, in these membrane models as a function of fluorescein-conjugated lipid architecture. Specific recognition and binding in this system are conveniently monitored by quenching of fluorescein emission upon penetration of fluorescein into the antibody's active site. Lipid structure was shown to play a large role in affecting antibody quenching. Interestingly, the observed degrees of quenching were nearly independent of the lipid membrane model studied, but directly correlated with the chemical structure of the lipids. In all cases, the antibody recognized and quenched most efficiently a lipid based on dioctadecylamine where fluorescein is attached to the headgroup via a long, flexible hydrophilic spacer. Dipalmitoyl phosphatidylethanolamine containing a fluorescein headgroup demonstrated only partial binding/quenching. Egg phosphatidylethanolamine with a fluorescein headgroup showed no susceptibility to antibody recognition, binding, or quenching. Formation of two-dimensional protein domains upon antibody binding to the fluorescein-lipids in monolayers is also presented. Chemical and physical requirements for these antibody-hapten complexes at membrane surfaces have been discussed in terms of molecular dynamics simulations based on recent crystallographic models for this antibody-hapten complex (Herron et al., 1989. Proteins Struct. Funct. Genet. 5:271-280).
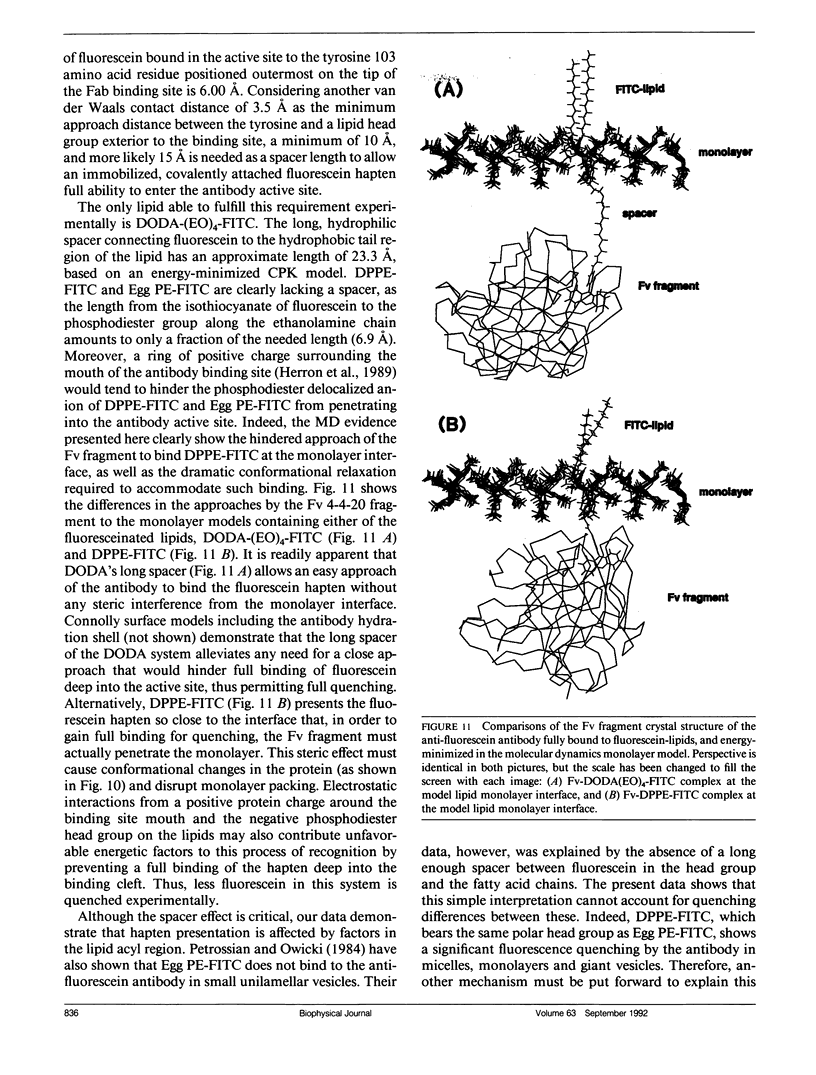
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates R. M., Ballard D. W., Voss E. W., Jr Comparative properties of monoclonal antibodies comprising a high-affinity anti-fluorescyl idiotype family. Mol Immunol. 1985 Aug;22(8):871–877. doi: 10.1016/0161-5890(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Bedzyk W. D., Johnson L. S., Riordan G. S., Voss E. W., Jr Comparison of variable region primary structures within an anti-fluorescein idiotype family. J Biol Chem. 1989 Jan 25;264(3):1565–1569. [PubMed] [Google Scholar]
- Blankenburg R., Meller P., Ringsdorf H., Salesse C. Interaction between biotin lipids and streptavidin in monolayers: formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry. 1989 Oct 3;28(20):8214–8221. doi: 10.1021/bi00446a037. [DOI] [PubMed] [Google Scholar]
- Darst S. A., Ahlers M., Meller P. H., Kubalek E. W., Blankenburg R., Ribi H. O., Ringsdorf H., Kornberg R. D. Two-dimensional crystals of streptavidin on biotinylated lipid layers and their interactions with biotinylated macromolecules. Biophys J. 1991 Feb;59(2):387–396. doi: 10.1016/S0006-3495(91)82232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber-Osguthorpe P., Roberts V. A., Osguthorpe D. J., Wolff J., Genest M., Hagler A. T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins. 1988;4(1):31–47. doi: 10.1002/prot.340040106. [DOI] [PubMed] [Google Scholar]
- Decher G., Ringsdorf H., Venzmer J., Bitter-Suermann D., Weisgerber C. Giant liposomes as model membranes for immunological studies: spontaneous insertion of purified K1-antigen (poly-alpha-2,8-NeuAc) of Escherichia coli. Biochim Biophys Acta. 1990 Apr 30;1023(3):357–364. doi: 10.1016/0005-2736(90)90127-a. [DOI] [PubMed] [Google Scholar]
- Gibson A. L., Herron J. N., He X. M., Patrick V. A., Mason M. L., Lin J. N., Kranz D. M., Voss E. W., Jr, Edmundson A. B. Differences in crystal properties and ligand affinities of an antifluorescyl Fab (4-4-20) in two solvent systems. Proteins. 1988;3(3):155–160. doi: 10.1002/prot.340030304. [DOI] [PubMed] [Google Scholar]
- Grainger D. W., Reichert A., Ringsdorf H., Salesse C. Hydrolytic action of phospholipase A2 in monolayers in the phase transition region: direct observation of enzyme domain formation using fluorescence microscopy. Biochim Biophys Acta. 1990 Apr 30;1023(3):365–379. doi: 10.1016/0005-2736(90)90128-b. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Herron J. N., He X. M., Mason M. L., Voss E. W., Jr, Edmundson A. B. Three-dimensional structure of a fluorescein-Fab complex crystallized in 2-methyl-2,4-pentanediol. Proteins. 1989;5(4):271–280. doi: 10.1002/prot.340050404. [DOI] [PubMed] [Google Scholar]
- Herron J. N., Kranz D. M., Jameson D. M., Voss E. W., Jr Thermodynamic properties of ligand binding by monoclonal anti-fluorescyl antibodies. Biochemistry. 1986 Aug 12;25(16):4602–4609. doi: 10.1021/bi00364a022. [DOI] [PubMed] [Google Scholar]
- Knight C. G., Stephens T. Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochem J. 1989 Mar 15;258(3):683–687. doi: 10.1042/bj2580683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz D. M., Herron J. N., Voss E. W., Jr Mechanisms of ligand binding by monoclonal anti-fluorescyl antibodies. J Biol Chem. 1982 Jun 25;257(12):6987–6995. [PubMed] [Google Scholar]
- Kranz D. M., Voss E. W., Jr Partial elucidation of an anti-hapten repertoire in BALB/c mice: comparative characterization of several monoclonal anti-fluorescyl antibodies. Mol Immunol. 1981 Oct;18(10):889–898. doi: 10.1016/0161-5890(81)90012-2. [DOI] [PubMed] [Google Scholar]
- Lasic D. D. The mechanism of vesicle formation. Biochem J. 1988 Nov 15;256(1):1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Nargessi R. D., Smith D. S. Fluorometric assays for avidin and biotin. Methods Enzymol. 1986;122:67–72. doi: 10.1016/0076-6879(86)22150-3. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L. Using molecular dynamics simulations on crambin to evaluate the suitability of different continuum dielectric and hydrogen atom models for protein simulations. J Biomol Struct Dyn. 1990 Apr;7(5):1019–1041. doi: 10.1080/07391102.1990.10508543. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Petrossian A., Kantor A. B., Owicki J. C. Synthesis and characterization of a highly fluorescent peptidyl-phosphatidylethanolamine. J Lipid Res. 1985 Jun;26(6):767–773. [PubMed] [Google Scholar]
- Petrossian A., Owicki J. C. Interaction of antibodies with liposomes bearing fluorescent haptens. Biochim Biophys Acta. 1984 Oct 3;776(2):217–227. doi: 10.1016/0005-2736(84)90211-6. [DOI] [PubMed] [Google Scholar]
- Reed R. A., Mattai J., Shipley G. G. Interaction of cholera toxin with ganglioside GM1 receptors in supported lipid monolayers. Biochemistry. 1987 Feb 10;26(3):824–832. doi: 10.1021/bi00377a025. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Ribi H. O., Ludwig D. S., Mercer K. L., Schoolnik G. K., Kornberg R. D. Three-dimensional structure of cholera toxin penetrating a lipid membrane. Science. 1988 Mar 11;239(4845):1272–1276. doi: 10.1126/science.3344432. [DOI] [PubMed] [Google Scholar]
- Ribi H. O., Reichard P., Kornberg R. D. Two-dimensional crystals of enzyme-effector complexes: ribonucleotide reductase at 18-A resolution. Biochemistry. 1987 Dec 1;26(24):7974–7979. doi: 10.1021/bi00398a064. [DOI] [PubMed] [Google Scholar]
- Stanton S. G., Kantor A. B., Petrossian A., Owicki J. C. Location and dynamics of a membrane-bound fluorescent hapten. A spectroscopic study. Biochim Biophys Acta. 1984 Oct 3;776(2):228–236. doi: 10.1016/0005-2736(84)90212-8. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Pagano R. E. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980 Jun 10;255(11):5404–5410. [PubMed] [Google Scholar]
- Swindlehurst C. A., Voss E. W., Jr Fluorescence measurements of immune complexes of Mab 4-4-20 with isomeric haptens. Biophys J. 1991 Mar;59(3):619–628. doi: 10.1016/S0006-3495(91)82277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E. Self-organization of IgE immunoglobulins on phospholipid films. Biochem J. 1987 Feb 15;242(1):293–296. doi: 10.1042/bj2420293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss E. W., Jr, Eschenfeldt W., Root R. T. Fluorescein: a complete antigenic group. Immunochemistry. 1976 May;13(5):447–453. doi: 10.1016/0019-2791(76)90382-7. [DOI] [PubMed] [Google Scholar]