Abstract
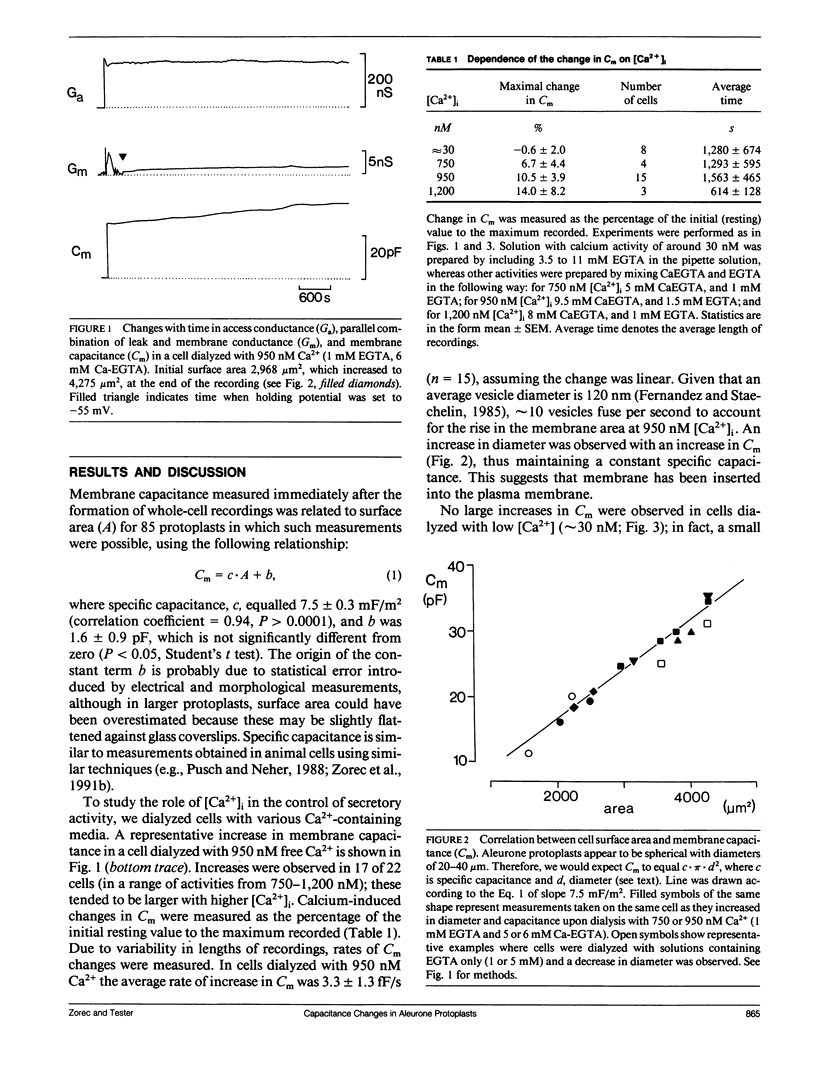
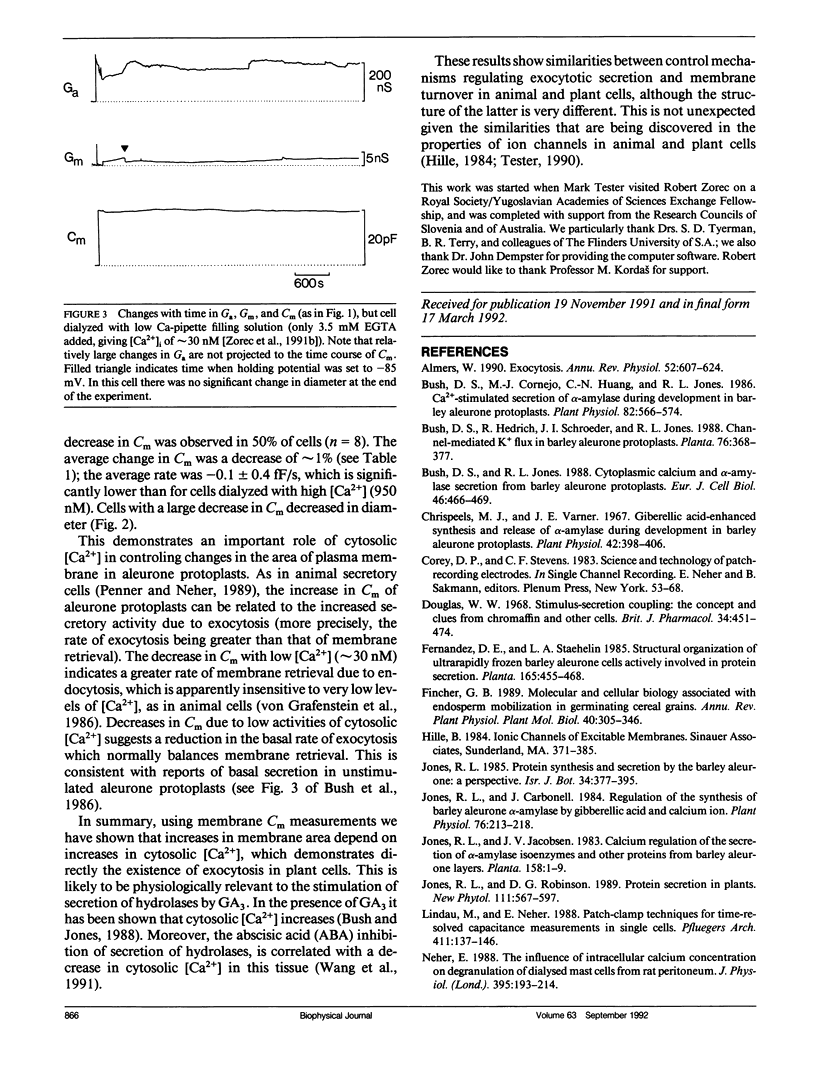
Although exocytosis is likely to occur in plant cells, the control of this process is the subject of speculation, as no direct measurements of vesicle fusion to the plasma membrane have been made. We used the patch clamp technique to monitor the secretory activity of single aleurone protoplasts by measuring membrane capacitance (Cm), while dialyzing the cytosol with different Ca2+ containing solutions. Secretory activity increased with [Ca2+]i ∼ 1 μM. This demonstrates directly the existence of exocytosis in plant cells, and suggests that both plant and animal cells share common mechanisms (cytosolic Ca2+) for the control of exocytotic secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Bush D. S., Cornejo M. J., Huang C. N., Jones R. L. Ca-stimulated secretion of alpha-amylase during development in barley aleurone protoplasts. Plant Physiol. 1986 Oct;82(2):566–574. doi: 10.1104/pp.82.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Carbonell J. Regulation of the synthesis of barley aleurone alpha-amylase by gibberellic Acid and calcium ions. Plant Physiol. 1984 Sep;76(1):213–218. doi: 10.1104/pp.76.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. The patch-clamp technique in the study of secretion. Trends Neurosci. 1989 Apr;12(4):159–163. doi: 10.1016/0166-2236(89)90059-3. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H., Roberts C. S., Baker P. F. Kinetic analysis of the triggered exocytosis/endocytosis secretory cycle in cultured bovine adrenal medullary cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2343–2352. doi: 10.1083/jcb.103.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]


