Abstract
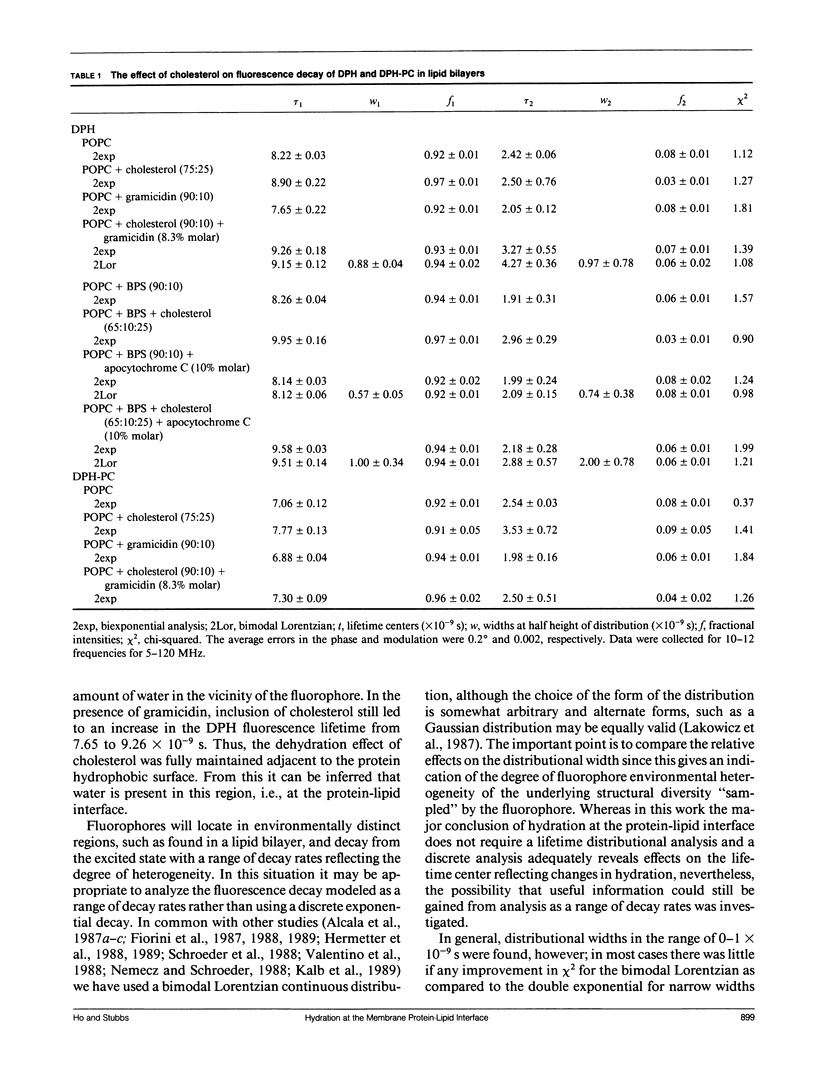
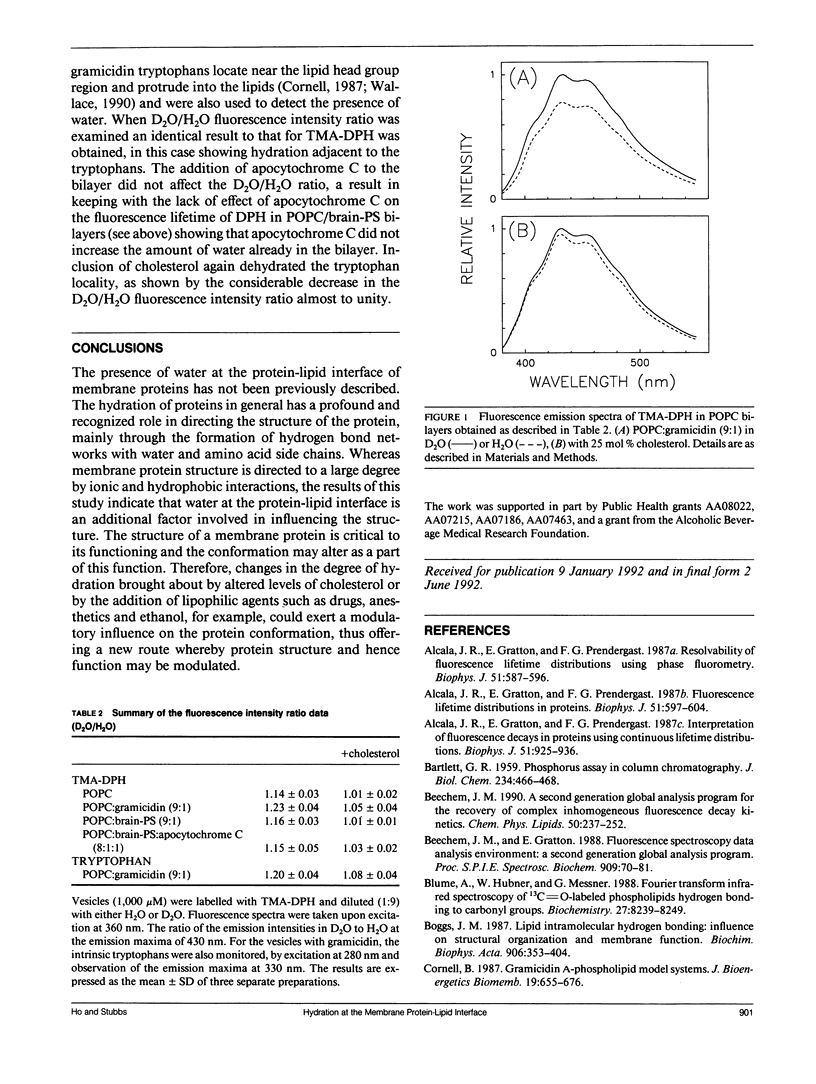
Evidence has been found for the existence water at the protein-lipid hydrophobic interface of the membrane proteins, gramicidin and apocytochrome C, using two related fluorescence spectroscopic approaches. The first approach exploited the fact that the presence of water in the excited state solvent cage of a fluorophore increases the rate of decay. For 1,6-diphenyl-1,3,5-hexatriene (DPH) and 1-palmitoyl-2-[[2-[4-(6-phenyl-trans-1,3,5- hexatrienyl)phenyl]ethyl]carbonyl]-3-sn-PC (DPH-PC), where the fluorophores are located in the hydrophobic core of the lipid bilayer, the introduction of gramicidin reduced the fluorescence lifetime, indicative of an increased presence of water in the bilayer. Since a high protein:lipid ratio was used, the fluorophores were forced to be adjacent to the protein hydrophobic surface, hence the presence of water in this region could be inferred. Cholesterol is known to reduce the water content of lipid bilayers and this effect was maintained at the protein-lipid interface with both gramicidin and apocytochrome C, again suggesting hydration in this region. The second approach was to use the fluorescence enhancement induced by exchanging deuterium oxide (D2O) for H2O. Both the fluorescence intensities of trimethylammonium-DPH, located in the lipid head group region, and of the gramicidin intrinsic tryptophans were greater in a D2O buffer compared with H2O, showing that the fluorophores were exposed to water in the bilayer at the protein-lipid interface. In the presence of cholesterol the fluorescence intensity ratio of D2O to H2O decreased, indicating a removal of water by the cholesterol, in keeping with the lifetime data. Altered hydration at the protein-lipid interface could affect conformation, thereby offering a new route by which membrane protein functioning may be modified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcala J. R., Gratton E., Prendergast F. G. Fluorescence lifetime distributions in proteins. Biophys J. 1987 Apr;51(4):597–604. doi: 10.1016/S0006-3495(87)83384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Interpretation of fluorescence decays in proteins using continuous lifetime distributions. Biophys J. 1987 Jun;51(6):925–936. doi: 10.1016/S0006-3495(87)83420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Resolvability of fluorescence lifetime distributions using phase fluorometry. Biophys J. 1987 Apr;51(4):587–596. doi: 10.1016/S0006-3495(87)83383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Beechem J. M. A second generation global analysis program for the recovery of complex inhomogeneous fluorescence decay kinetics. Chem Phys Lipids. 1989 Jun;50(3-4):237–251. doi: 10.1016/0009-3084(89)90052-2. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Cornell B. Gramicidin A--phospholipid model systems. J Bioenerg Biomembr. 1987 Dec;19(6):655–676. doi: 10.1007/BF00762301. [DOI] [PubMed] [Google Scholar]
- Davenport L., Dale R. E., Bisby R. H., Cundall R. B. Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985 Jul 16;24(15):4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- Fiorini R. M., Valentino M., Glaser M., Gratton E., Curatola G. Fluorescence lifetime distributions of 1,6-diphenyl-1,3,5-hexatriene reveal the effect of cholesterol on the microheterogeneity of erythrocyte membrane. Biochim Biophys Acta. 1988 Apr 22;939(3):485–492. doi: 10.1016/0005-2736(88)90095-8. [DOI] [PubMed] [Google Scholar]
- Fiorini R., Gratton E., Curatola G. Effect of cholesterol on membrane microheterogeneity: a study using 1,6-diphenyl-1,3,5-hexatriene fluorescence lifetime distributions. Biochim Biophys Acta. 1989 Nov 28;1006(2):198–202. doi: 10.1016/0005-2760(89)90196-3. [DOI] [PubMed] [Google Scholar]
- Fiorini R., Valentino M., Wang S., Glaser M., Gratton E. Fluorescence lifetime distributions of 1,6-diphenyl-1,3,5-hexatriene in phospholipid vesicles. Biochemistry. 1987 Jun 30;26(13):3864–3870. doi: 10.1021/bi00387a019. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton E., Limkeman M. A continuously variable frequency cross-correlation phase fluorometer with picosecond resolution. Biophys J. 1983 Dec;44(3):315–324. doi: 10.1016/S0006-3495(83)84305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Methods Enzymol. 1983;97:261–274. doi: 10.1016/0076-6879(83)97138-0. [DOI] [PubMed] [Google Scholar]
- Ho C., Williams B. W., Stubbs C. D. Analysis of cell membrane micro-heterogeneity using the fluorescence lifetime of DPH-type fluorophores. Biochim Biophys Acta. 1992 Mar 2;1104(2):273–282. doi: 10.1016/0005-2736(92)90041-j. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- Kalb E., Paltauf F., Hermetter A. Fluorescence lifetime distributions of diphenylhexatriene-labeled phosphatidylcholine as a tool for the study of phospholipid-cholesterol interactions. Biophys J. 1989 Dec;56(6):1245–1253. doi: 10.1016/S0006-3495(89)82771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Cherek H., Gryczynski I., Joshi N., Johnson M. L. Analysis of fluorescence decay kinetics measured in the frequency domain using distributions of decay times. Biophys Chem. 1987 Oct;28(1):35–50. doi: 10.1016/0301-4622(87)80073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P. Construction and performance of a variable-frequency phase-modulation fluorometer. Biophys Chem. 1985 Jan;21(1):61–78. doi: 10.1016/0301-4622(85)85007-9. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Burgess S. W. A dimerization model for the concentration dependent photophysical properties of diphenylhexatriene and its phospholipid derivatives. DPHpPC and DPHpPA. Biophys J. 1989 Oct;56(4):723–733. doi: 10.1016/S0006-3495(89)82720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGrasso P. V., Moll F., 3rd, Cross T. A. Solvent history dependence of gramicidin A conformations in hydrated lipid bilayers. Biophys J. 1988 Aug;54(2):259–267. doi: 10.1016/S0006-3495(88)82955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz G., Schroeder F. Time-resolved fluorescence investigation of membrane cholesterol heterogeneity and exchange. Biochemistry. 1988 Oct 4;27(20):7740–7749. doi: 10.1021/bi00420a024. [DOI] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Rusch R. M., Gratton E. A photophysical model for diphenylhexatriene fluorescence decay in solvents and in phospholipid vesicles. Biophys J. 1991 Feb;59(2):466–475. doi: 10.1016/S0006-3495(91)82240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A., Jordi W., de Kruijff B. Studies on the lipid dependency and mechanism of the translocation of the mitochondrial precursor protein apocytochrome c across model membranes. J Biol Chem. 1986 Mar 15;261(8):3846–3856. [PubMed] [Google Scholar]
- Schroeder F., Nemecz G., Gratton E., Barenholz Y., Thompson T. E. Fluorescence properties of cholestatrienol in phosphatidylcholine bilayer vesicles. Biophys Chem. 1988 Oct;32(1):57–72. doi: 10.1016/0301-4622(88)85034-8. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Latorre R. Influence of cholesterol on water penetration into bilayers. Science. 1982 Apr 2;216(4541):65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- Straume M., Litman B. J. Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry. 1987 Aug 11;26(16):5121–5126. doi: 10.1021/bi00390a034. [DOI] [PubMed] [Google Scholar]
- Teeter M. M. Water-protein interactions: theory and experiment. Annu Rev Biophys Biophys Chem. 1991;20:577–600. doi: 10.1146/annurev.bb.20.060191.003045. [DOI] [PubMed] [Google Scholar]
- Valentino M., Governa M., Gratton E., Fiorini R., Curatola G., Bertoli E. Increased membrane heterogeneity in stimulated human granulocytes. FEBS Lett. 1988 Jul 18;234(2):451–454. doi: 10.1016/0014-5793(88)80136-4. [DOI] [PubMed] [Google Scholar]
- Wallace B. A. Gramicidin channels and pores. Annu Rev Biophys Biophys Chem. 1990;19:127–157. doi: 10.1146/annurev.bb.19.060190.001015. [DOI] [PubMed] [Google Scholar]
- Williams B. W., Scotto A. W., Stubbs C. D. Effect of proteins on fluorophore lifetime heterogeneity in lipid bilayers. Biochemistry. 1990 Apr 3;29(13):3248–3255. doi: 10.1021/bi00465a016. [DOI] [PubMed] [Google Scholar]
- Williams B. W., Stubbs C. D. Properties influencing fluorophore lifetime distributions in lipid bilayers. Biochemistry. 1988 Oct 18;27(21):7994–7999. doi: 10.1021/bi00421a004. [DOI] [PubMed] [Google Scholar]
- Worcester D. L., Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutrol diffraction. J Mol Biol. 1976 Jan 25;100(3):359–378. doi: 10.1016/s0022-2836(76)80068-x. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]


