Abstract
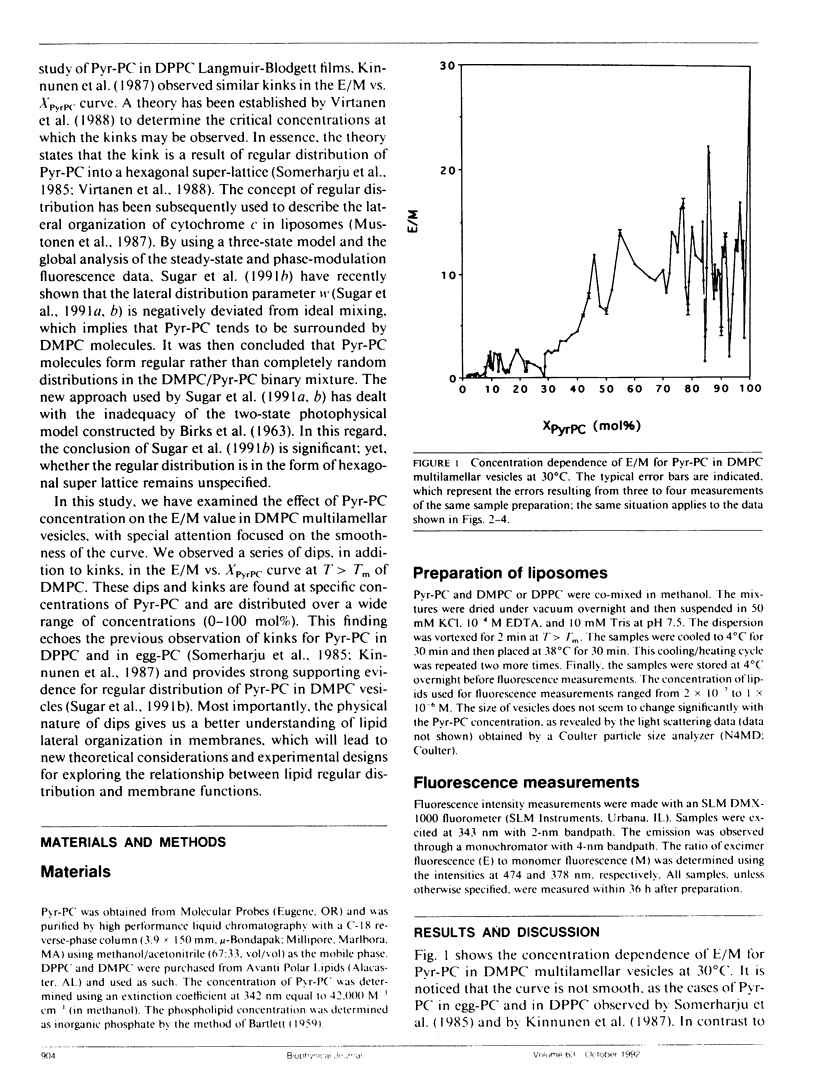
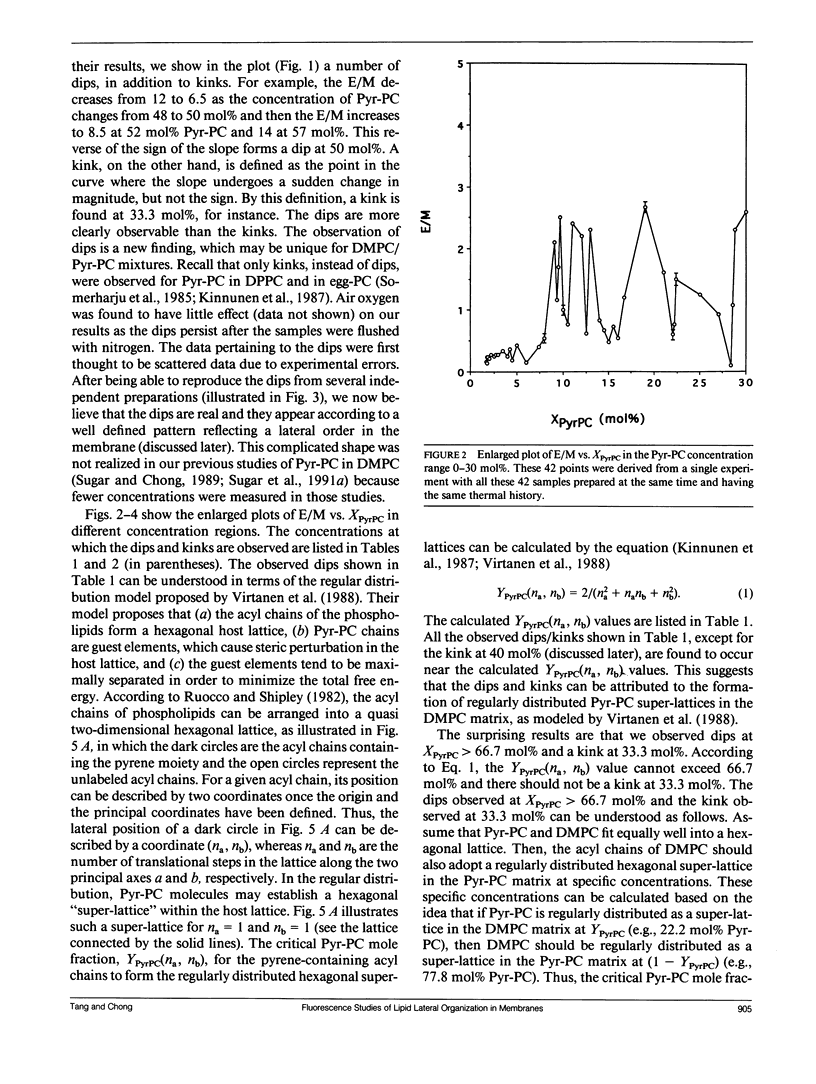
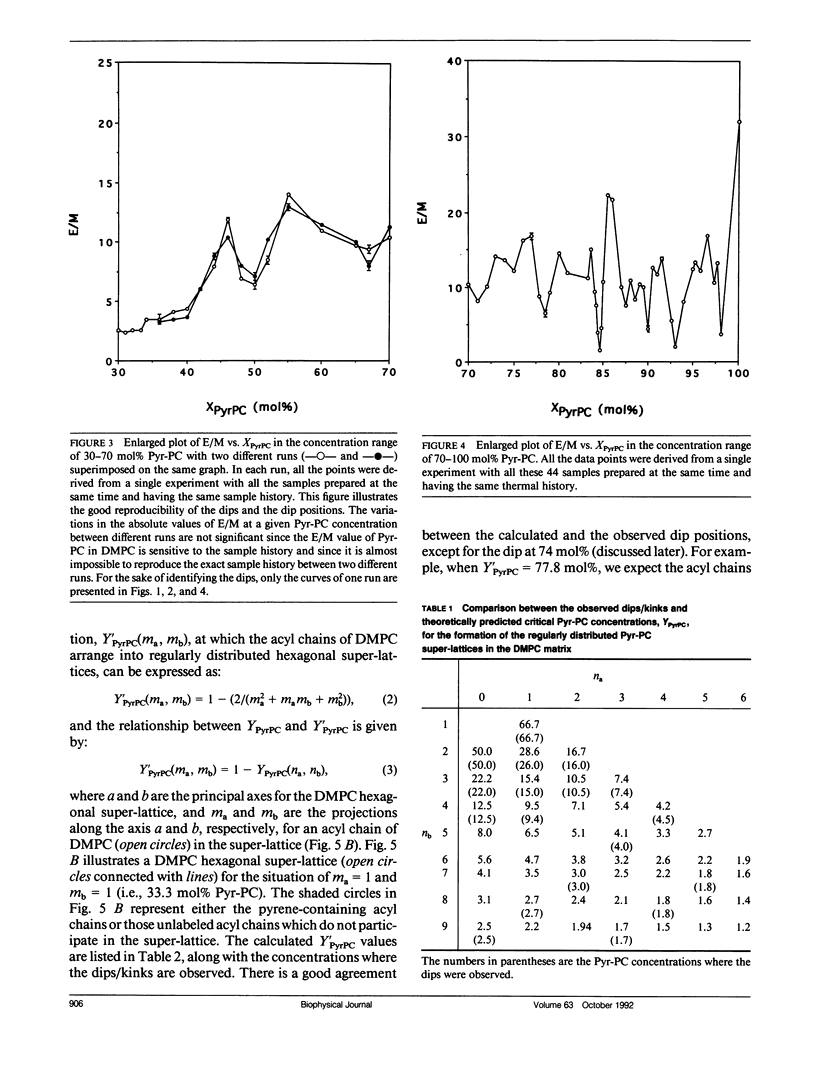
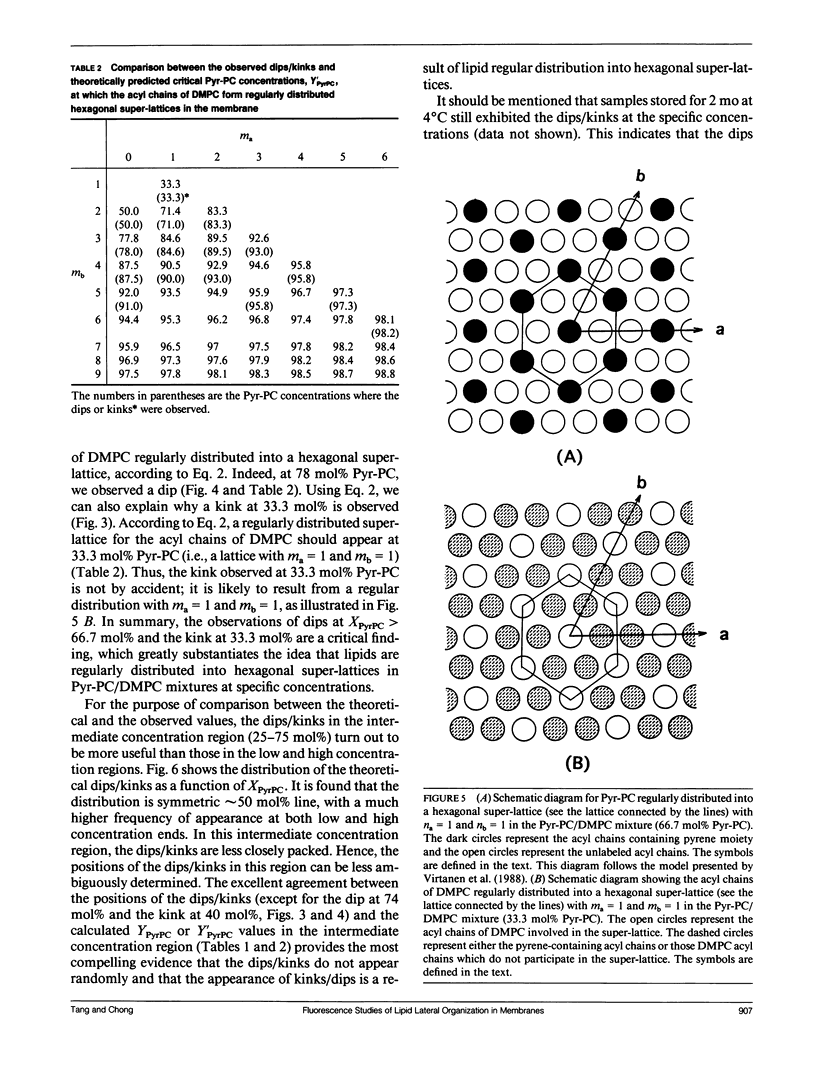
We have examined the effect of 1-palmitoyl-2-(10-pyrenyl)decanoyl-sn-glycerol-3-phosphatidylcholine (Pyr-PC) concentration on the ratio of excimer fluorescence to monomer fluorescence (E/M) in L-alpha-dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles at 30 degrees C, with special attention focussed on the smoothness of the curve. We observed a series of dips, in addition to kinks, in the plot of E/M versus the mole fraction of Pyr-PC (XPyrPC). The observation of dips is a new finding, perhaps unique for Pyr-PC in DMPC since only kinks were observed for Pyr-PC in L-alpha-dipalmitoylphosphatidylcholine (DPPC) and in egg yolk phosphatidylcholine (egg-PC) (Somerharju et al., 1985. Biochemistry. 24: 2773-2781). The dips/kinks observed here are distributed according to a well defined pattern reflecting a lateral order in the membrane, and distributed symmetrically with respect to 50 mol% Pyr-PC. Some of the dips appear at specific concentrations (YPyrPC) according to the hexagonal super-lattice model proposed by Virtanen et al. (1988. J. Mol. Electr. 4: 233-236). However, the observations of dips at XPyrPC > 66.7 mol% and the kink at 33.3 mol% cannot be interpreted by the model of Virtanen et al. (1988). These surprising results can be understood by virtue of an extended hexagonal super-lattice model, in which we have proposed that if the pyrene-containing acyl chains are regularly distributed as a hexagonal super-lattice in the DMPC matrix at a specific concentration YPyrPC, then the acyl chains of DMPC can form a regularly distributed hexagonal super-lattice in the membrane at a critical concentration (1-YPyrPC). The excellent agreement between the calculated and the observed dip/kink positions, except for the dip at 74 mol% and the kink at 40 mol%, provides most compelling evidence that lipids are regularly distributed into hexagonal super-lattices in Pyr-PC/DMPC mixtures at specific concentrations. The physical nature of the dips not only gives us a better understanding of lipid lateral organization in membranes but also will lead to new theoretical considerations and experimental designs for exploring the relationship between lipid regular distribution and membrane functions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berclaz T., McConnell H. M. Phase Equilibria in binary mixtures of dimyristoylphosphatidylcholine and cardiolipin. Biochemistry. 1981 Nov 10;20(23):6635–6640. doi: 10.1021/bi00526a018. [DOI] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Barber J. Evidence that pyrene excimer formation in membranes is not diffusion-controlled. Biochim Biophys Acta. 1986 Jun 26;858(2):221–234. doi: 10.1016/0005-2736(86)90327-5. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Thompson T. E. Oxygen quenching of pyrene-lipid fluorescence in phosphatidylcholine vesicles. A probe for membrane organization. Biophys J. 1985 May;47(5):613–621. doi: 10.1016/S0006-3495(85)83957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Visualization of Ca2+-induced phospholipid domains. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4475–4479. doi: 10.1073/pnas.84.13.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko R. C., Sugár I. P., Barenholz Y., Thompson T. E. Lateral distribution of a pyrene-labeled phosphatidylcholine in phosphatidylcholine bilayers: fluorescence phase and modulation study. Biochemistry. 1986 Jul 1;25(13):3813–3823. doi: 10.1021/bi00361a012. [DOI] [PubMed] [Google Scholar]
- Jan N., Lookman T., Pink D. A. On computer simulation methods used to study models of two-component lipid bilayers. Biochemistry. 1984 Jul 3;23(14):3227–3231. doi: 10.1021/bi00309a017. [DOI] [PubMed] [Google Scholar]
- Jones M. E., Lentz B. R. Phospholipid lateral organization in synthetic membranes as monitored by pyrene-labeled phospholipids: effects of temperature and prothrombin fragment 1 binding. Biochemistry. 1986 Feb 11;25(3):567–574. doi: 10.1021/bi00351a009. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991 Mar;57(2-3):375–399. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Melchior D. L. Lipid domains in fluid membranes: a quick-freeze differential scanning calorimetry study. Science. 1986 Dec 19;234(4783):1577–1580. doi: 10.1126/science.3787264. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman M. A., Thompson T. E. Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry. 1980 Feb 5;19(3):439–444. doi: 10.1021/bi00544a006. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Vauhkonen M., Perry D., Eisinger J. Lateral diffusivity of lipid analogue excimeric probes in dimyristoylphosphatidylcholine bilayers. Biophys J. 1990 Feb;57(2):281–290. doi: 10.1016/S0006-3495(90)82530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Thompson T. E., Allietta M., Brown R. E., Johnson M. L., Tillack T. W. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1985 Jul 25;817(2):229–237. doi: 10.1016/0005-2736(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Wong M., Allietta M., Thompson T. E. Organization of the glycosphingolipid asialo-GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1982 Oct 7;691(2):261–273. doi: 10.1016/0005-2736(82)90415-1. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- von Dreele P. H. Estimation of lateral species separation from phase transitions in nonideal two-dimensional lipid mixtures. Biochemistry. 1978 Sep 19;17(19):3939–3943. doi: 10.1021/bi00612a009. [DOI] [PubMed] [Google Scholar]


