Abstract
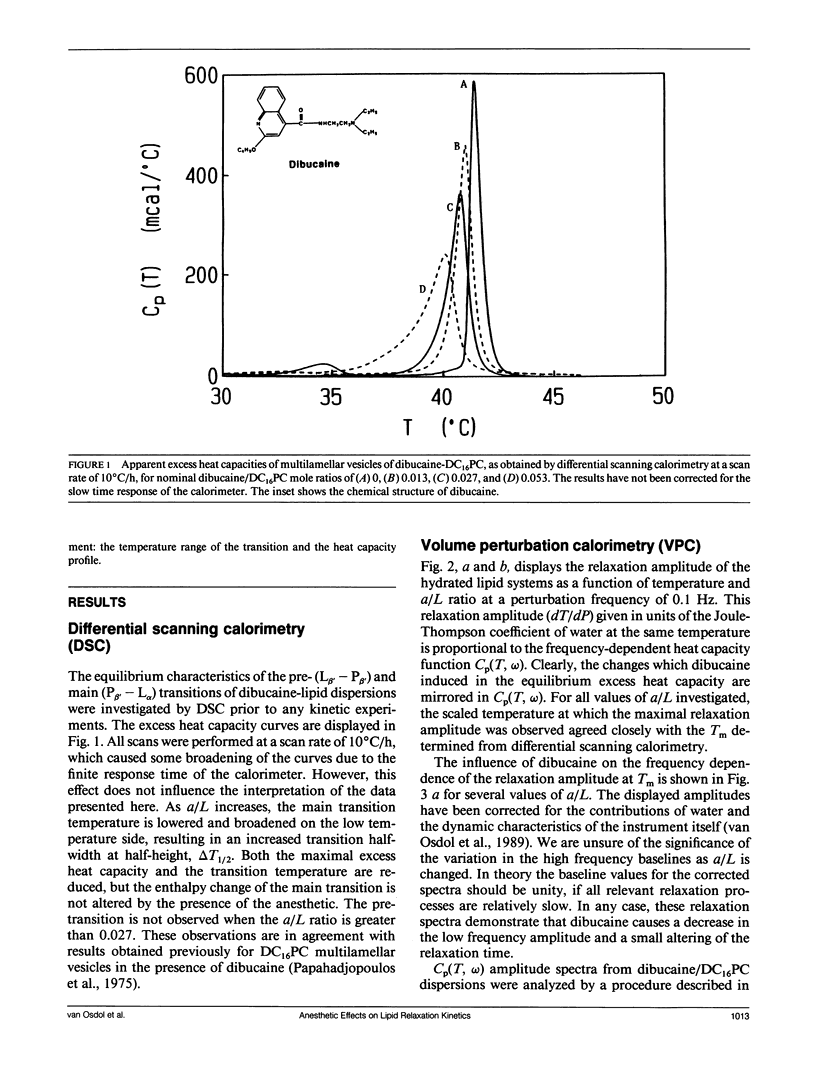
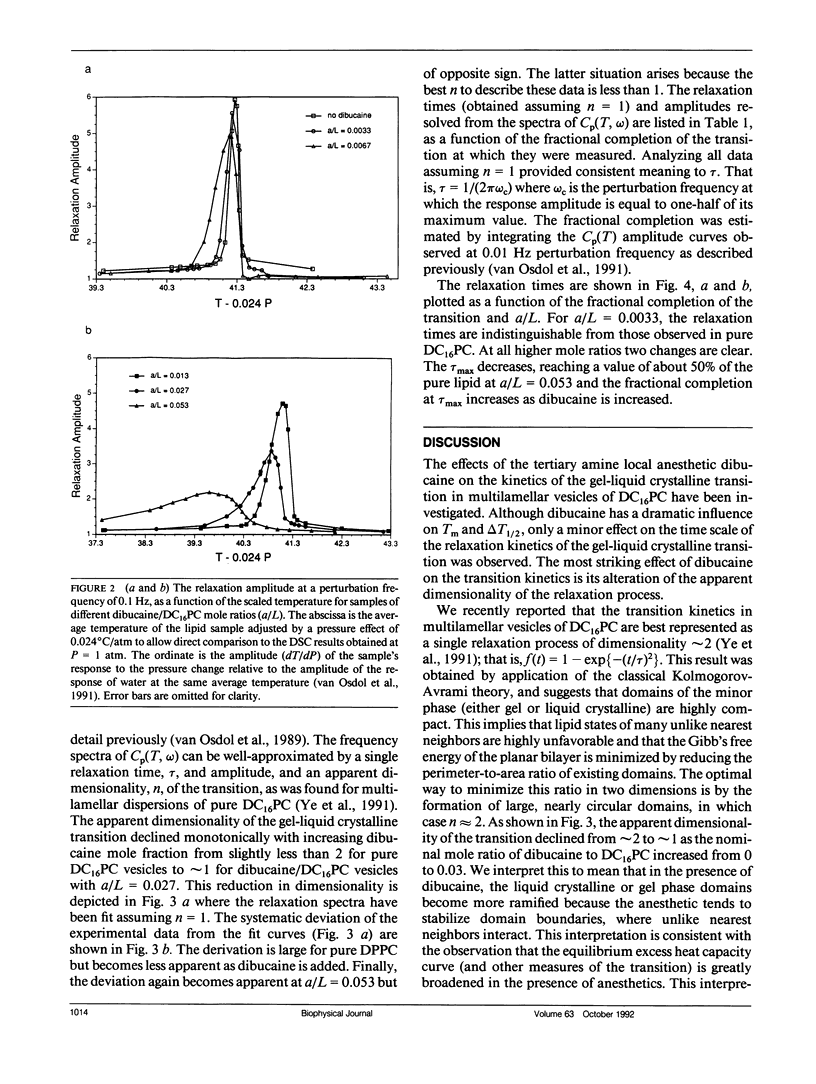
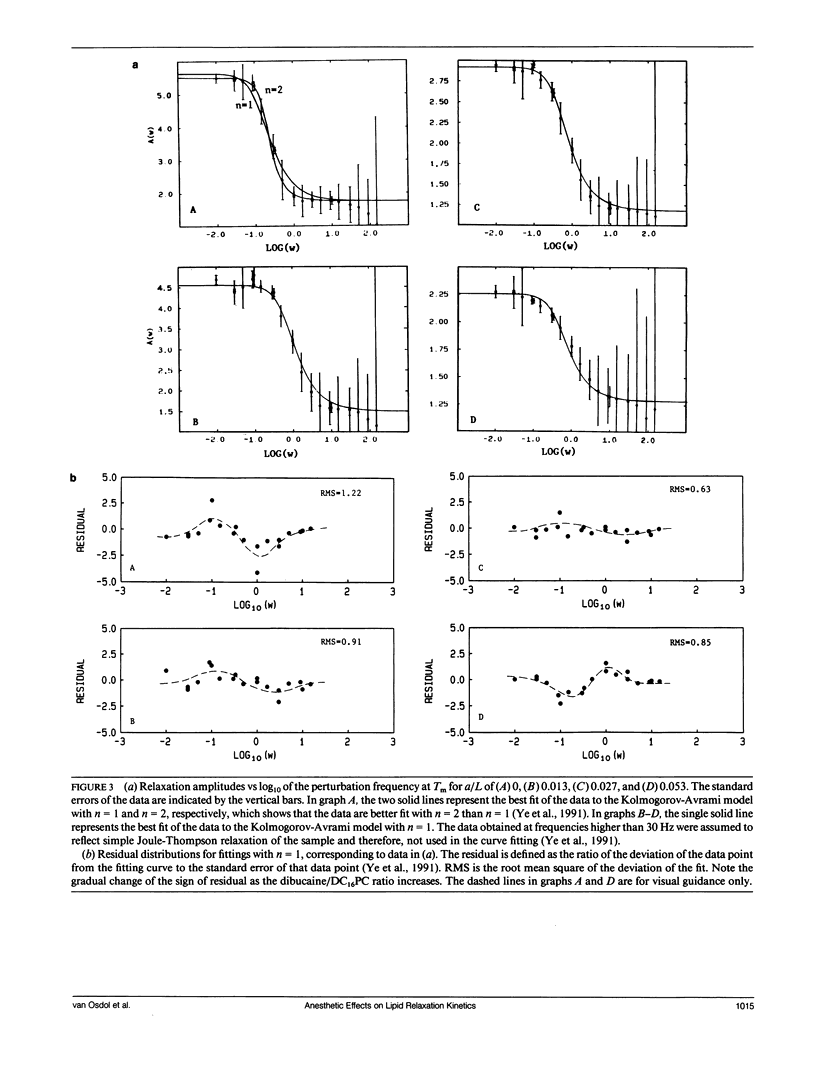
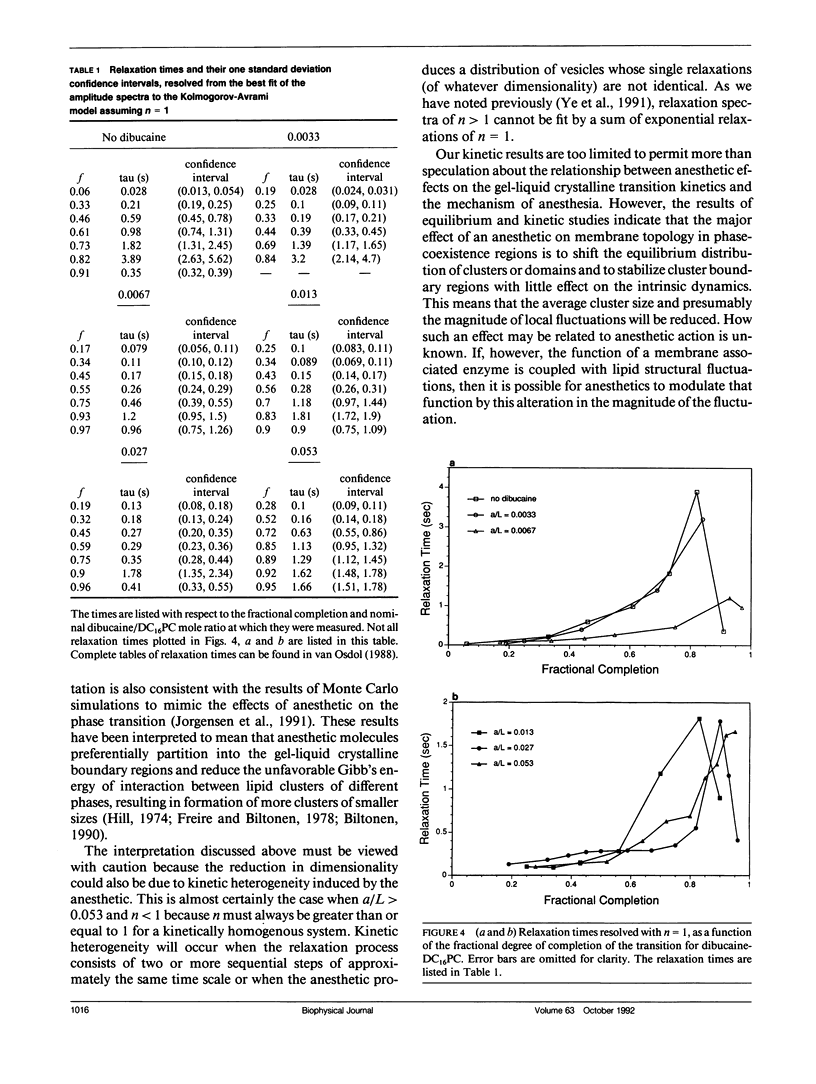
The effects of the anesthetic dibucaine on the relaxation kinetics of the gel-liquid crystalline transition of dipalmitoylphosphatidylcholine (DC16PC) multilamellar vesicles have been investigated using volume-perturbation calorimetry. The temperature and pressure responses to a periodic volume perturbation were measured in real time. Data collected in the time domain were subsequently converted into and analyzed in the frequency domain using Fourier series representations of the perturbation and response functions. The Laplace transform of the classical Kolmogorov-Avrami kinetic relation was employed to describe the relaxation dynamics in the frequency domain. The relaxation time of anesthetic-lipid mixtures, as a function of the fractional degree of melting, appears to be qualitatively similar to that of pure lipid systems, with a pronounced maximum, tau max, observed at a temperature corresponding to greater than 75% melting. The tau max decreases by a factor of approximately 2 as the nominal anesthetic/lipid mole ratio increases from 0 to 0.013 and exhibits no further change as the nominal anesthetic/lipid mole ratio is increased. However, the fractional dimensionality of the relaxation process decreases monotonically from slightly less than two to approximately one as the anesthetic/lipid mole ratio increases from 0 to 0.027. At higher ratios, the dimensionality appears to be less than one. These results are interpreted in terms of the classical kinetic theory and related to those obtained from Monte Carlo simulations. Specifically, low concentrations of dibucaine appear to reduce the average cluster size and cause the fluctuating lipid clusters to become more ramified. At the highest concentration of dibucaine, where n < 1, the system must be kinetically heterogeneous.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eftink M. R., Puri R. K., Ghahramani M. D. Local anesthetic-phospholipid interactions. The pH dependence of the binding of dibucaine to dimyristoylphosphatidylcholine vesicles. Biochim Biophys Acta. 1985 Feb 28;813(1):137–140. doi: 10.1016/0005-2736(85)90354-2. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire E., Biltonen R. Estimation of molecular averages and equilibrium fluctuations in lipid bilayer systems from the excess heat capacity function. Biochim Biophys Acta. 1978 Dec 4;514(1):54–68. doi: 10.1016/0005-2736(78)90076-7. [DOI] [PubMed] [Google Scholar]
- Hill M. W. The effect of anaesthetic-like molecules on the phase transition in smectic mesophases of dipalmitoyllecithin. I. The normal alcohol up to C equals 9 and three inhalation anaesthetics. Biochim Biophys Acta. 1974 Jul 12;356(1):117–124. doi: 10.1016/0005-2736(74)90299-5. [DOI] [PubMed] [Google Scholar]
- Jørgensen K., Ipsen J. H., Mouritsen O. G., Bennett D., Zuckermann M. J. The effects of density fluctuations on the partitioning of foreign molecules into lipid bilayers: application to anaesthetics and insecticides. Biochim Biophys Acta. 1991 Aug 26;1067(2):241–253. doi: 10.1016/0005-2736(91)90050-i. [DOI] [PubMed] [Google Scholar]
- Kamaya H., Ueda I., Moore P. S., Eyring H. Antagonism between high pressure and anesthetics in the thermal phase-transition of dipalmitoyl phosphatidylcholine bilayer. Biochim Biophys Acta. 1979 Jan 5;550(1):131–137. doi: 10.1016/0005-2736(79)90121-4. [DOI] [PubMed] [Google Scholar]
- Macdonald A. G. A dilatometric investigation of the effects of general anaesthetics, alcohols and hydrostatic pressure on the phase transition in smectic mesophases of dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1978 Feb 2;507(1):26–37. doi: 10.1016/0005-2736(78)90371-1. [DOI] [PubMed] [Google Scholar]
- Mountcastle D. B., Biltonen R. L., Halsey M. J. Effect of anesthetics and pressure on the thermotropic behavior of multilamellar dipalmitoylphosphatidylcholine liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4906–4910. doi: 10.1073/pnas.75.10.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Poste G., Shepherd G. Effects of local anesthetics on membrane properties. I. Changes in the fluidity of phospholipid bilayers. Biochim Biophys Acta. 1975 Jul 18;394(4):504–519. doi: 10.1016/0005-2736(75)90137-6. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Lentz B. R., Barenholz Y., Biltonen R. L., Thompson T. E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976 Apr 6;15(7):1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- Trudell J. R., Payan D. G., Chin J. H., Cohen E. N. The antagonistic effect of an inhalation anesthetic and high pressure on the phase diagram of mixed dipalmitoyl-dimyristoylphosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1975 Jan;72(1):210–213. doi: 10.1073/pnas.72.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Greenberg M., Kanehisa M. I. Anesthetic action of membrane lipids. Biochemistry. 1977 Jul 12;16(14):3115–3121. doi: 10.1021/bi00633a012. [DOI] [PubMed] [Google Scholar]
- Van Osdol W. W., Biltonen R. L., Johnson M. L. Measuring the kinetics of membrane phase transitions. J Biochem Biophys Methods. 1989;20(1):1–46. doi: 10.1016/0165-022x(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Yang CP, Nagle JF. Phase transformations in lipids follow classical kinetics with small fractional dimensionalities. Phys Rev A Gen Phys. 1988 May 15;37(10):3993–4000. doi: 10.1103/physreva.37.3993. [DOI] [PubMed] [Google Scholar]
- Ye Q., van Osdol W. W., Biltonen R. L. Gel-liquid crystalline transition of some multilamellar lipid bilayers follows classical kinetics with a fractional dimensionality of approximately two. Biophys J. 1991 Nov;60(5):1002–1007. doi: 10.1016/S0006-3495(91)82137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Osdol W. W., Johnson M. L., Ye Q., Biltonen R. L. Relaxation dynamics of the gel to liquid-crystalline transition of phosphatidylcholine bilayers. Effects of chainlength and vesicle size. Biophys J. 1991 Apr;59(4):775–785. doi: 10.1016/S0006-3495(91)82290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


