Abstract
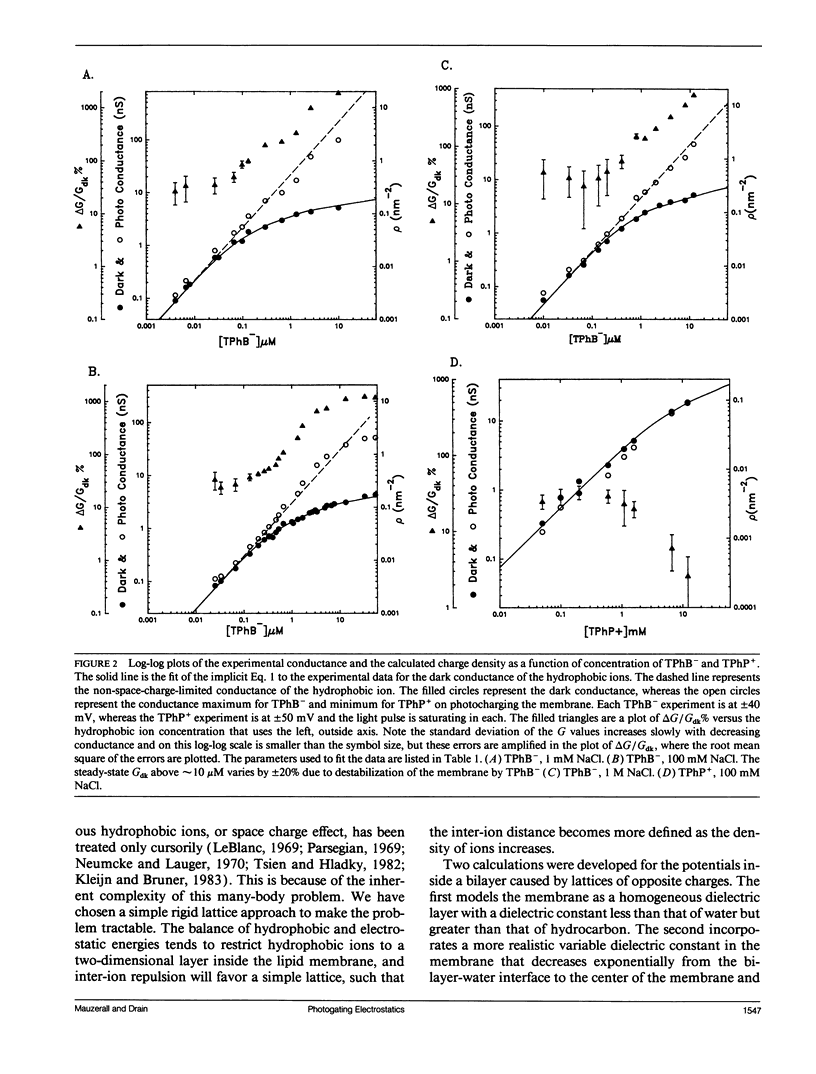
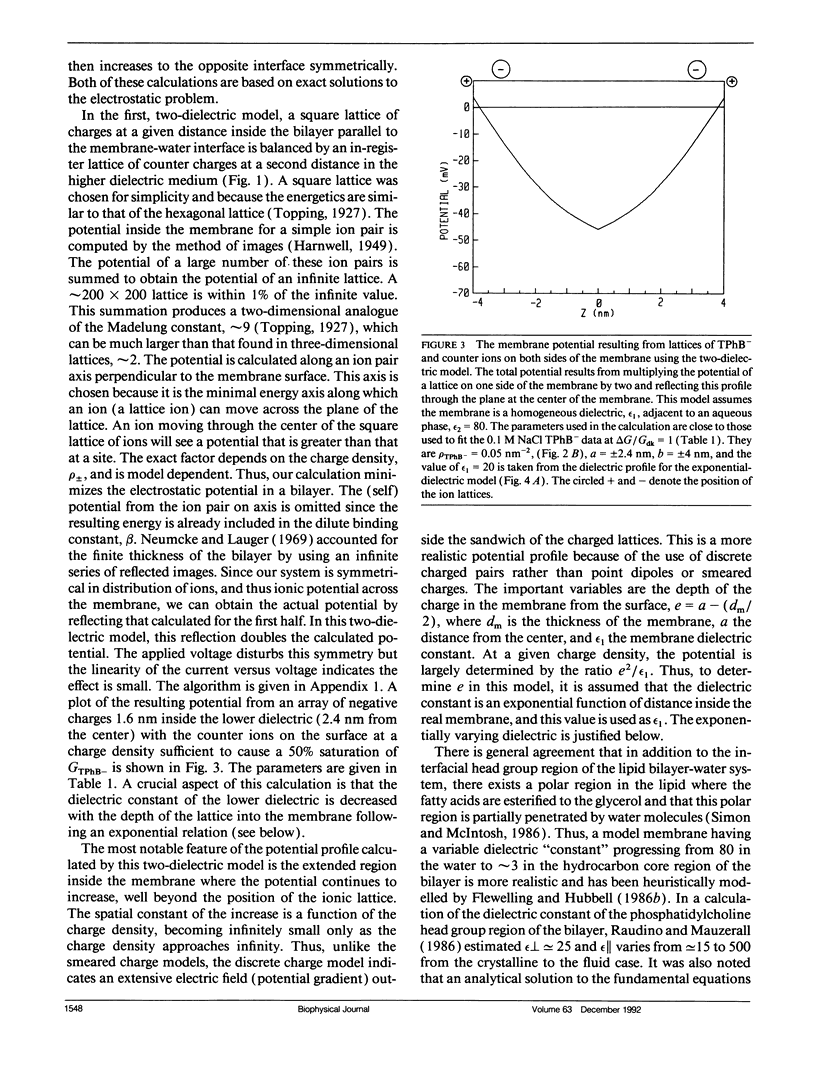
The conductances of the lipophilic ions tetraphenylboride and tetraphenylphosphonium across a lipid bilayer can be increased or decreased, i.e., gated, by the photoformation of closed-shell metalloporphyrin cations within the bilayer. The gating can be effected by pulsed or continuous light or by chemical oxidants. At high concentrations of lipophilic anions where the dark conductance is saturated due to space charge in the bilayer, the photogated conductance can increase 15-fold. The formation of porphyrin cations allows the conductance to increase to its nonspace charge limited value. Conversely, the decrease of conductance in the light of phosphonium cations diminishes toward zero as the dark conductance becomes space charge limited. We present electrostatic models of the space charge limited conductance that accurately fit the data. One model includes an exponentially varying dielectric constant for the polar regions of the bilayer that allows an analytical solution to the electrostatic problem. The exponential variation of the dielectric constant effectively screens the potential and implies that the inside and outside of real dielectric interfaces can be electrically isolated from one another. The charge density, the distance into the membrane of the ions, about one-quarter of its thickness, and the dielectric constant at that position are determined by these models. These calculations indicate that there is insufficient porphyrin charge density to cancel the boride ion space charge and the following article proposes a novel ion chain mechanism to explain these effects. These models indicate that the positive potential arising from oriented carbonyl ester groups, previously used to explain the 10(3)-fold larger conductance of hydrophobic anions over cations, is smaller than previously estimated. However, the synergistic movement of the positive choline group into the membrane can account for the large positive potential.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Gisin B. F. Influence of membrane structure on ion transport through lipid bilayer membranes. J Membr Biol. 1978 Jun 9;40(4):293–314. doi: 10.1007/BF01874161. [DOI] [PubMed] [Google Scholar]
- Braun H. P. The transport of hydrophobic ions across lipid bilayers. Biochim Biophys Acta. 1987 Oct 2;903(2):292–302. doi: 10.1016/0005-2736(87)90219-7. [DOI] [PubMed] [Google Scholar]
- Bäuerle H. D., Seelig J. Interaction of charged and uncharged calcium channel antagonists with phospholipid membranes. Binding equilibrium, binding enthalpy, and membrane location. Biochemistry. 1991 Jul 23;30(29):7203–7211. doi: 10.1021/bi00243a023. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Hsu K., Chapman D. Polarised absorption spectroscopy of chlorophyll-lipid membranes. Biochim Biophys Acta. 1972 Jun 23;267(3):512–522. doi: 10.1016/0005-2728(72)90179-x. [DOI] [PubMed] [Google Scholar]
- Drain C. M., Christensen B., Mauzerall D. Photogating of ionic currents across a lipid bilayer. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6959–6962. doi: 10.1073/pnas.86.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain C. M., Mauzerall D. C. Photogating of ionic currents across lipid bilayers. Hydrophobic ion conductance by an ion chain mechanism. Biophys J. 1992 Dec;63(6):1556–1563. doi: 10.1016/S0006-3495(92)81739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellena J. F., Dominey R. N., Archer S. J., Xu Z. C., Cafiso D. S. Localization of hydrophobic ions in phospholipid bilayers using 1H nuclear Overhauser effect spectroscopy. Biochemistry. 1987 Jul 14;26(14):4584–4592. doi: 10.1021/bi00388a062. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys J. 1986 Feb;49(2):531–540. doi: 10.1016/S0006-3495(86)83663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K., Ruston D., Zimmerberg J., Parsegian V. A., Rand R. P., Fuller N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J. 1992 May;61(5):1213–1223. doi: 10.1016/S0006-3495(92)81931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Ilani A., Liu T. M., Mauzerall D. The effect of oxygen on the amplitude of photodriven electron transfer across the lipid bilayer-water interface. Biophys J. 1985 May;47(5):679–684. doi: 10.1016/S0006-3495(85)83964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani A., Mauzerall D. The potential span of photoredox reactions of porphyrins and chlorophyll at the lipid bilayer-water interface. Biophys J. 1981 Jul;35(1):79–92. doi: 10.1016/S0006-3495(81)84775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn W. B., Bruner L. J. The influence of hydrophobic ions and dipolar molecules on the electrostatic barrier in biomembranes. J Theor Biol. 1983 Jan 7;100(1):139–152. doi: 10.1016/0022-5193(83)90098-x. [DOI] [PubMed] [Google Scholar]
- Le Blanc O. H., Jr Tetraphenylborate conductance through lipid bilayer membranes. Biochim Biophys Acta. 1969;193(2):350–360. doi: 10.1016/0005-2736(69)90195-3. [DOI] [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Läuger P. Nonlinear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations. Biophys J. 1969 Sep;9(9):1160–1170. doi: 10.1016/S0006-3495(69)86443-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- Raudino A., Mauzerall D. Dielectric properties of the polar head group region of zwitterionic lipid bilayers. Biophys J. 1986 Sep;50(3):441–449. doi: 10.1016/S0006-3495(86)83480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchelli F., Jori G., Gobbo S., Tronchin M. Liposomes as models to study the distribution of porphyrins in cell membranes. Biochim Biophys Acta. 1991 May 31;1065(1):42–48. doi: 10.1016/0005-2736(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Scherer P. G., Seelig J. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 1989 Sep 19;28(19):7720–7728. doi: 10.1021/bi00445a030. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Hladky S. B. Ion repulsion within membranes. Biophys J. 1982 Jul;39(1):49–56. doi: 10.1016/S0006-3495(82)84489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodle M. C., Mauzerall D. Photoinitiated ion movements in bilayer membranes containing magnesium octaethylporphyrin. Biophys J. 1986 Sep;50(3):431–439. doi: 10.1016/S0006-3495(86)83479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodle M., Zhang J. W., Mauzerall D. Kinetics of charge transfer at the lipid bilayer-water interface on the nanosecond time scale. Biophys J. 1987 Oct;52(4):577–586. doi: 10.1016/S0006-3495(87)83247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai G., Büldt G., Seelig A., Seelig J. Neutron diffraction studies on phosphatidylcholine model membranes. II. Chain conformation and segmental disorder. J Mol Biol. 1979 Nov 15;134(4):693–706. doi: 10.1016/0022-2836(79)90480-7. [DOI] [PubMed] [Google Scholar]
- Zheng C., Vanderkooi G. Molecular origin of the internal dipole potential in lipid bilayers: calculation of the electrostatic potential. Biophys J. 1992 Oct;63(4):935–941. doi: 10.1016/S0006-3495(92)81673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



