Abstract
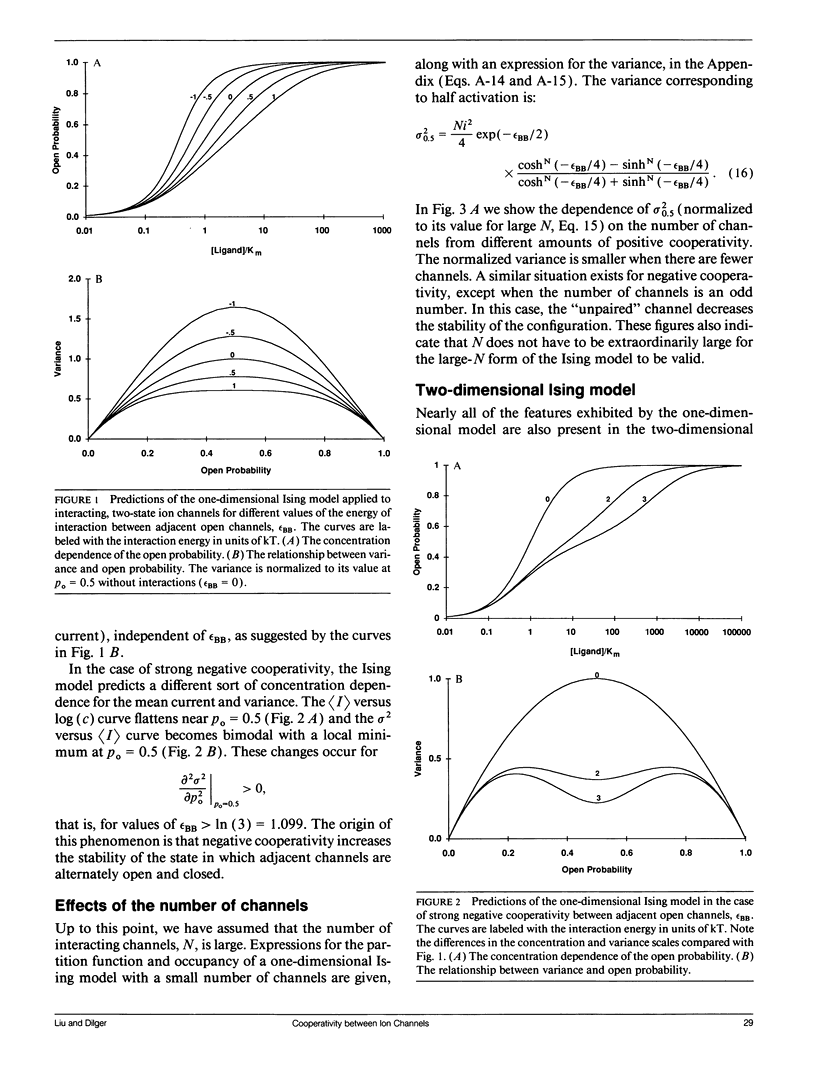
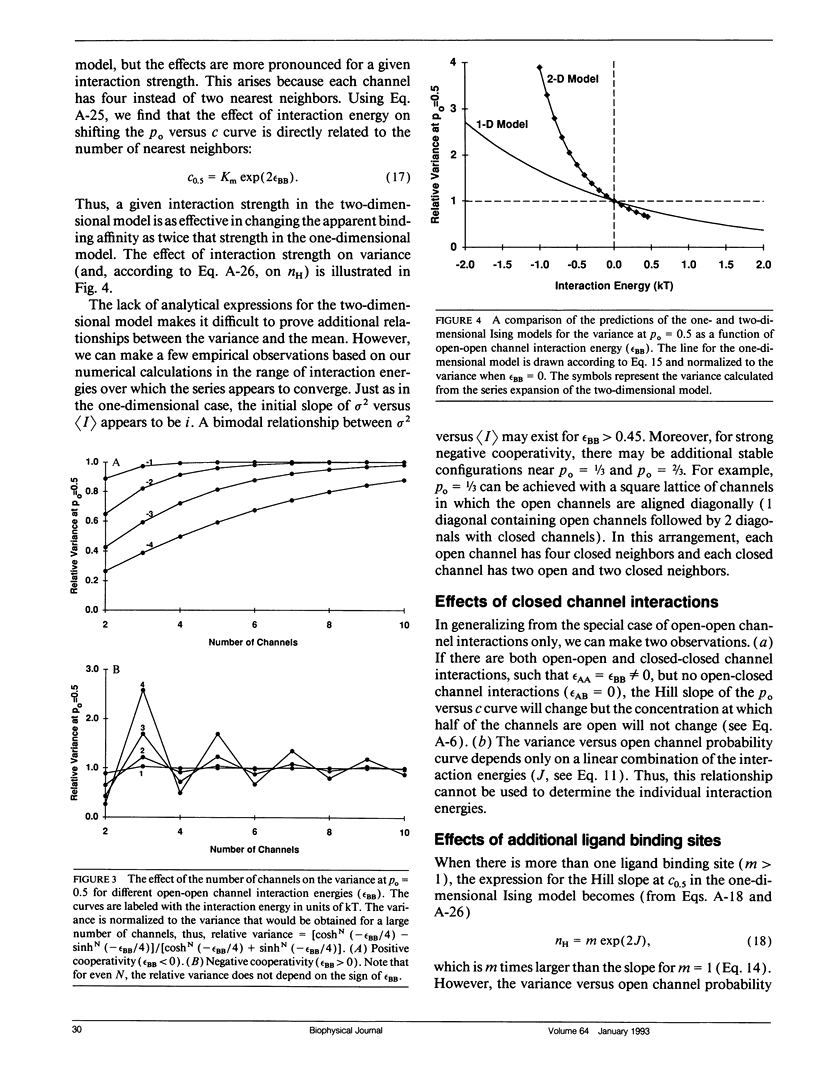
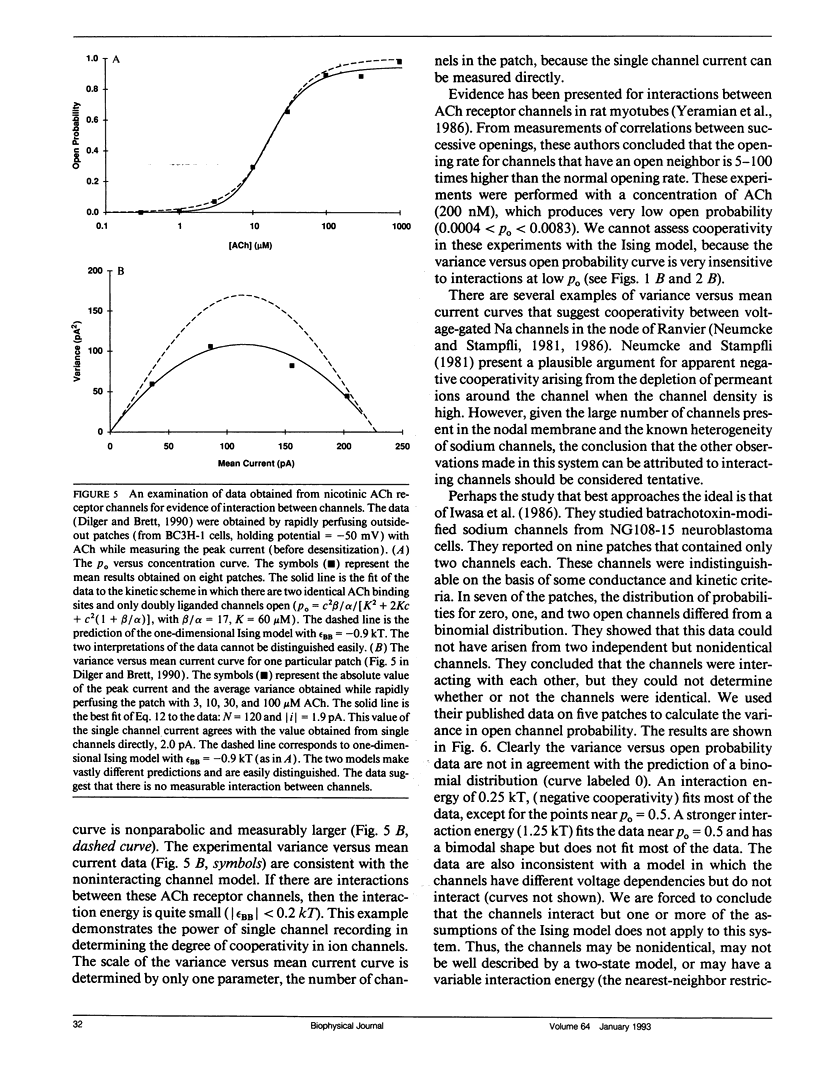
The Ising model of statistical physics provides a framework for studying systems of protomers in which nearest neighbors interact with each other. In this article, the Ising model is applied to the study of cooperative phenomena between ligand-gated ion channels. Expressions for the mean open channel probability, rho o, and the variance, sigma 2, are derived from the grand partition function. In the one-dimensional Ising model, interactions between neighboring open channels give rise to a sigmoidal rho o versus concentration curve and a nonquadratic relationship between sigma 2 and rho o. Positive cooperativity increases the slope at the midpoint of the rho o versus concentration curve, shifts the apparent binding affinity to lower concentrations, and increases the variance for a given rho o. Negative cooperativity has the opposite effects. Strong negative cooperativity results in a bimodal sigma 2 versus rho o curve. The slope of the rho o versus concentration curve increases linearly with the number of binding sites on a protomer, but the sigma 2 versus rho o relationship is independent of the number of ligand binding sites. Thus, the sigma 2 versus rho o curve provides unambiguous information about channel interactions. In the two-dimensional Ising model, rho o and sigma 2 are calculated numerically from a series expansion of the grand partition function appropriate for weak interactions. Virtually all of the features exhibited by the one-dimensional model are qualitatively present in the two-dimensional model. These models are also applicable to voltage-gated ion channels.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990 Apr;57(4):723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. On the theory of ion transport across the nerve membrane. II. Potassium ion kinetics and cooperativity (with x = 4). Proc Natl Acad Sci U S A. 1971 Aug;68(8):1711–1715. doi: 10.1073/pnas.68.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. Three-state, steady-state Ising systems: Monte Carlo and Bragg-Williams treatments. Proc Natl Acad Sci U S A. 1981 Jan;78(1):4–8. doi: 10.1073/pnas.78.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K., Ehrenstein G., Moran N., Jia M. Evidence for interactions between batrachotoxin-modified channels in hybrid neuroblastoma cells. Biophys J. 1986 Sep;50(3):531–537. doi: 10.1016/S0006-3495(86)83491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Kinetics of unliganded acetylcholine receptor channel gating. Biophys J. 1986 Mar;49(3):663–672. doi: 10.1016/S0006-3495(86)83693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Nagy K. Interaction between sodium channels in mouse neuroblastoma cells. Eur Biophys J. 1985;12(1):13–18. doi: 10.1007/BF00254090. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Liu Y., Dilger J. P. Opening rate of acetylcholine receptor channels. Biophys J. 1991 Aug;60(2):424–432. doi: 10.1016/S0006-3495(91)82068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron. 1989 Mar;2(3):1195–1205. doi: 10.1016/0896-6273(89)90304-8. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Alteration of the conductance of Na+ channels in the nodal membrane of frog nerve by holding potential and tetrodotoxin. Biochim Biophys Acta. 1983 Jan 5;727(1):177–184. doi: 10.1016/0005-2736(83)90382-6. [DOI] [PubMed] [Google Scholar]
- Pauling L. The Oxygen Equilibrium of Hemoglobin and Its Structural Interpretation. Proc Natl Acad Sci U S A. 1935 Apr;21(4):186–191. doi: 10.1073/pnas.21.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The conductance of sodium channels under conditions of reduced current at the node of Ranvier. J Physiol. 1980 Oct;307:131–142. doi: 10.1113/jphysiol.1980.sp013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Acetylcholine receptor activation by a site-selective ligand: nature of brief open and closed states in BC3H-1 cells. J Physiol. 1986 Jan;370:357–379. doi: 10.1113/jphysiol.1986.sp015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J Biol Chem. 1980 Nov 10;255(21):10144–10156. [PubMed] [Google Scholar]
- Thompson C. J. Models for hemoglobin and allosteric enzymes. Biopolymers. 1968;6(8):1101–1118. doi: 10.1002/bip.1968.360060806. [DOI] [PubMed] [Google Scholar]
- Yeramian E., Trautmann A., Claverie P. Acetylcholine receptors are not functionally independent. Biophys J. 1986 Aug;50(2):253–263. doi: 10.1016/S0006-3495(86)83459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


