Abstract
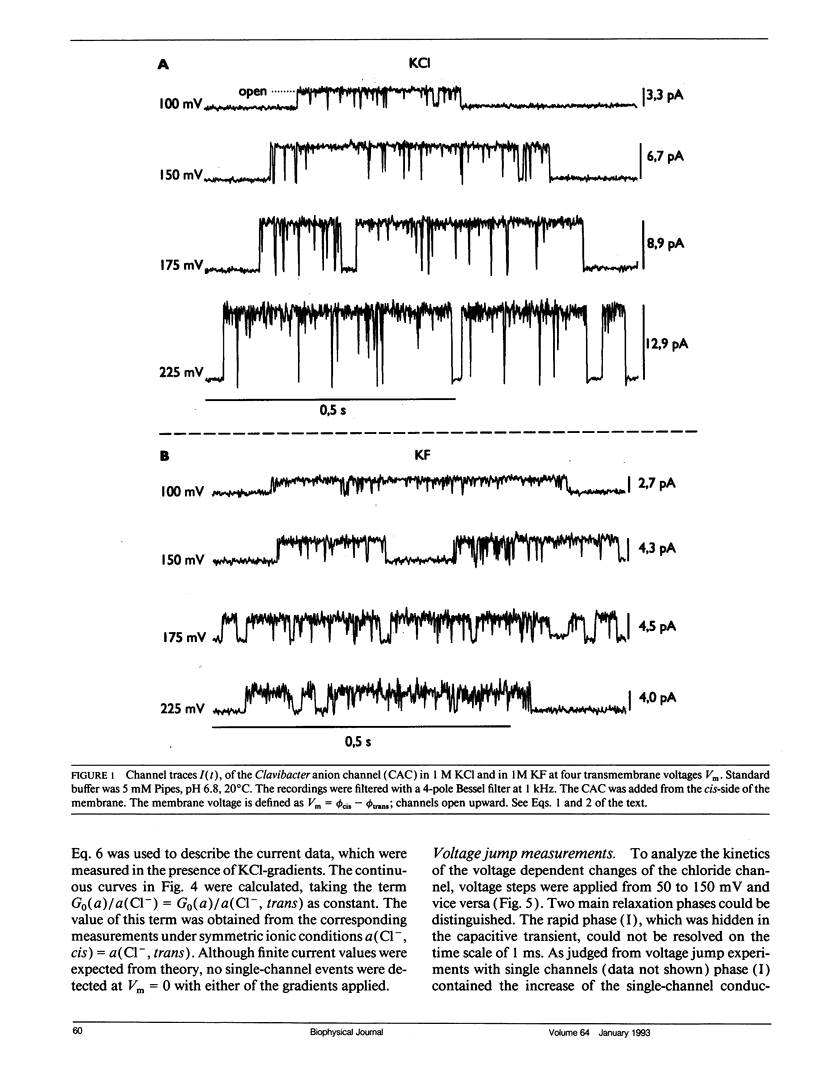
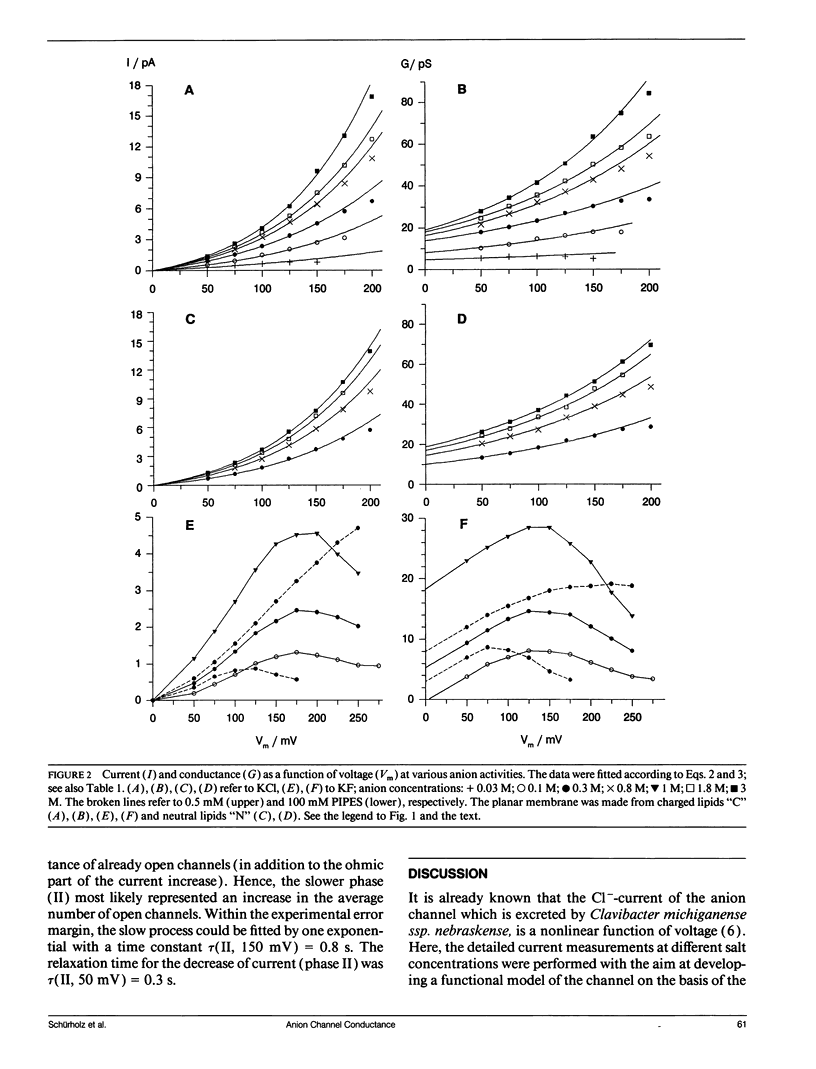
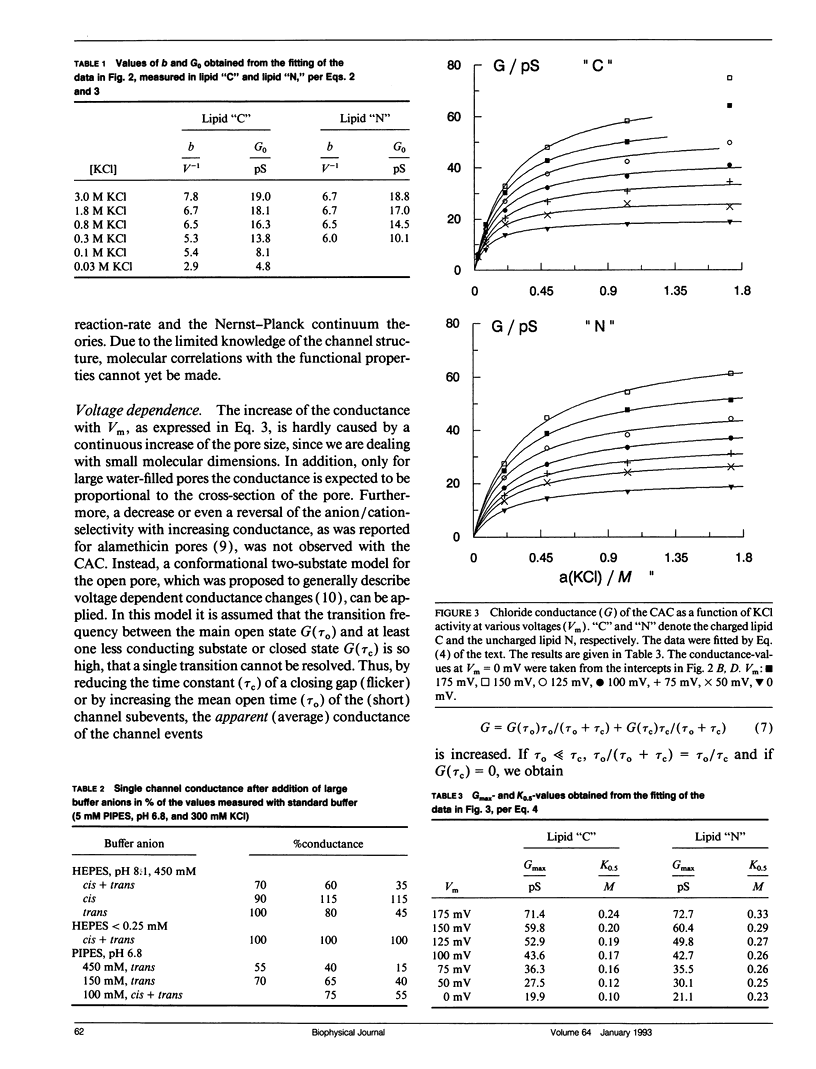
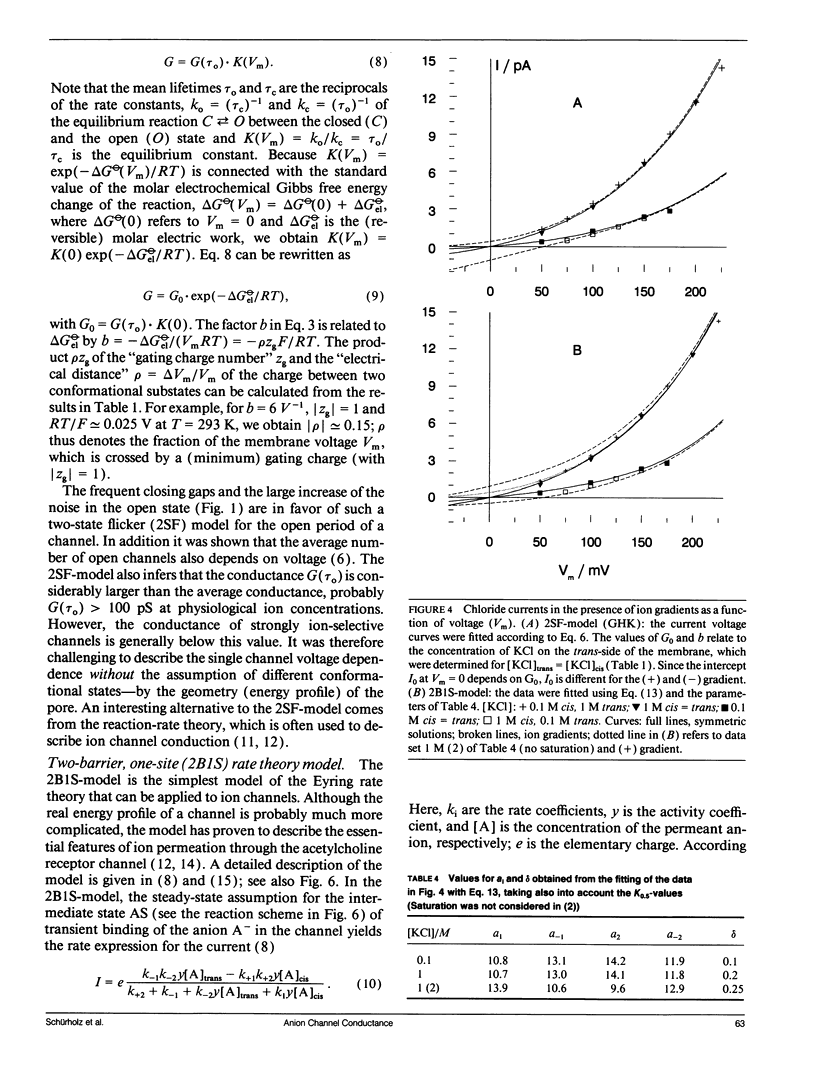
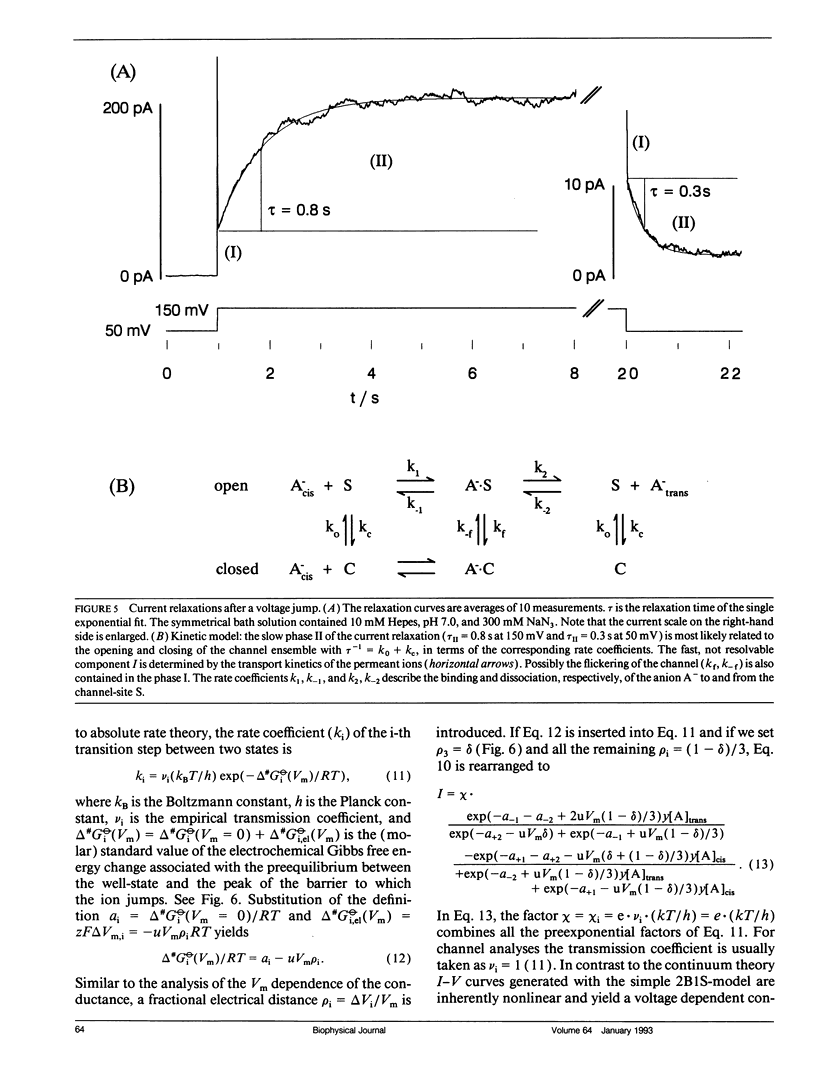
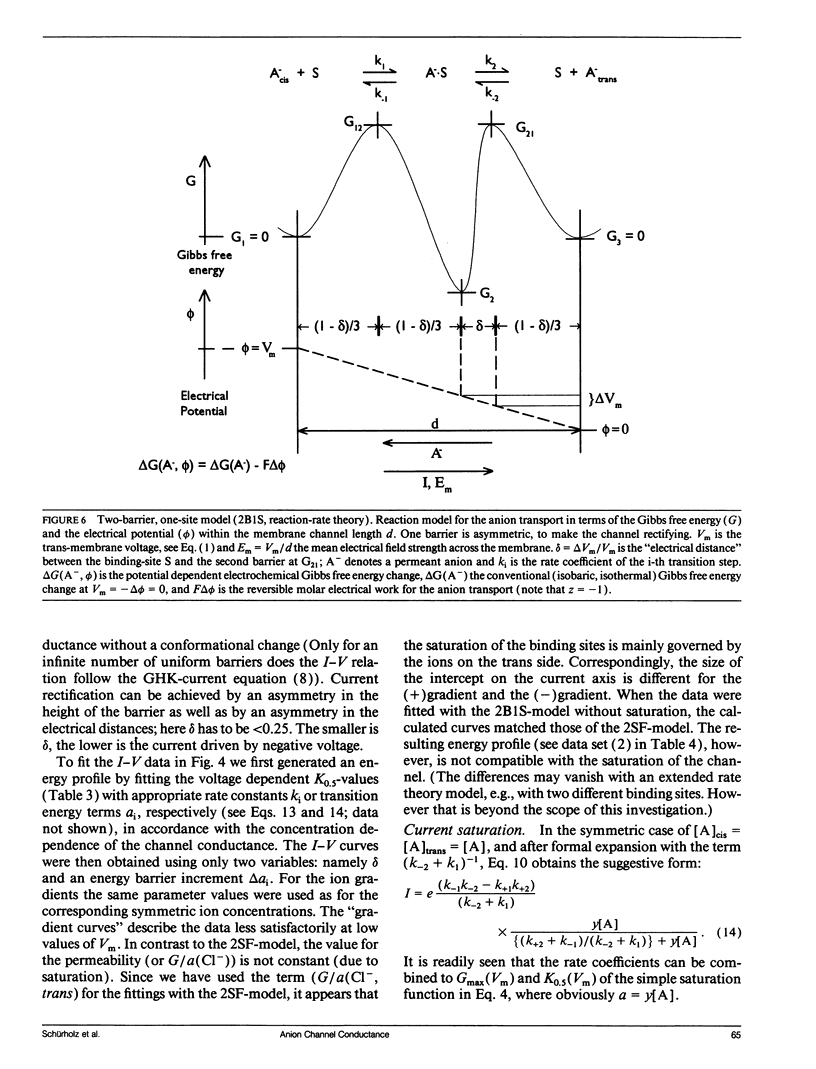
The anion channel protein from Clavibacter michiganense ssp. nebraskense (Schürholz, Th. et al. 1991, J. Membrane Biol. 123: 1-8) was analyzed at different concentrations of KCl and KF. At 0.8 M KCl the conductance G(Vm) increases exponentially from 21 pS at 50 mV up to 53 pS at Vm = 200 mV, 20°C. The concentration dependence of G(Vm) corresponds to a Michaelis-Menten type saturation function at all membrane voltage values applied (0-200 mV). The anion concentration K0.5, where G(Vm) has its half-maximum value, increases from 0.12 M at 50 mV to 0.24 M at 175 mV for channels in a soybean phospholipid bilayer. The voltage dependence of the single channel conductance, which is different for charged and neutral lipid bilayers, can be described either by a two-state flicker (2SF) model and the Nernst-Planck continuum theory, or by a two barrier, one-site (2B1S) model with asymmetric barriers. The increase in the number of open channels after a voltage jump from 50 mV to 150 mV has a time constant of 0.8 s. The changes of the single-channel conductance are much faster (<1 ms). The electric part of the gating process is characterized by the (reversible) molar electrical work ΔGθel = ρZgFVm ≈ -1.3 RT, which corresponds to the movement of one charge of the gating charge number ǀZgǀ = 1 across the fraction ρ = ΔVm/Vm = 0.15 of the membrane voltage Vm = 200 mV. Unlike with chloride, the single channel conductance of fluoride has a maximum at about 150 mV in the presence of the buffer PIPES (≥5 mM, pH 6.8) with K0.5 ≈ 1 M. It is shown that the decrease in conductance is due to a blocking of the channel by the PIPES anion. In summary, the results indicate that the anion transport by the Clavibacter anion channel (CAC) does not require a voltage dependent conformation change of the CAC.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dani J. A., Eisenman G. Acetylcholine-activated channel current-voltage relations in symmetrical na solutions. Biophys J. 1984 Jan;45(1):10–12. doi: 10.1016/S0006-3495(84)84087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophys J. 1986 Mar;49(3):607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Levitt D. G. Diffusion and kinetic approaches to describe permeation in ionic channels. J Theor Biol. 1990 Oct 7;146(3):289–301. doi: 10.1016/s0022-5193(05)80740-4. [DOI] [PubMed] [Google Scholar]
- Hanke W., Boheim G. The lowest conductance state of the alamethicin pore. Biochim Biophys Acta. 1980 Mar 13;596(3):456–462. doi: 10.1016/0005-2736(80)90134-0. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Sigworth F. J. Open channel noise. IV. Estimation of rapid kinetics of formamide block in gramicidin A channels. Biophys J. 1988 Oct;54(4):757–764. doi: 10.1016/S0006-3495(88)83013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Interpretation of biological ion channel flux data--reaction-rate versus continuum theory. Annu Rev Biophys Biophys Chem. 1986;15:29–57. doi: 10.1146/annurev.bb.15.060186.000333. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ionic channels with conformational substates. Biophys J. 1985 May;47(5):581–590. doi: 10.1016/S0006-3495(85)83954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schürholz T., Wilimzig M., Katsiou E., Eichenlaub R. Anion channel forming activity from the plant pathogenic bacterium Clavibacter michiganense ssp. nebraskense. J Membr Biol. 1991 Jul;123(1):1–8. doi: 10.1007/BF01993957. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys J. 1985 May;47(5):709–720. doi: 10.1016/S0006-3495(85)83968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P., Stynes B. A., Coackley W., Yeoh G. T., Petterson D. S. Glycolipid toxins from parasitised annual ryegrass: a comparison with tunicamycin. Biochem Biophys Res Commun. 1982 Apr 14;105(3):835–840. doi: 10.1016/0006-291x(82)91045-2. [DOI] [PubMed] [Google Scholar]


