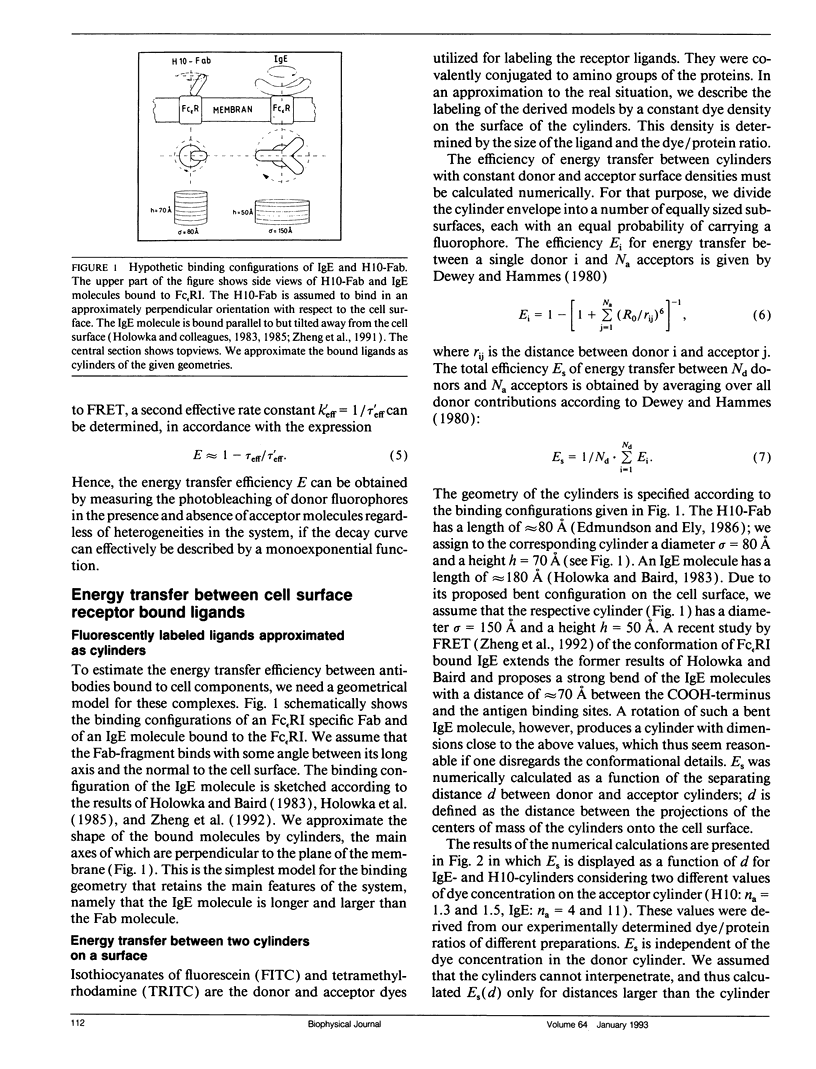
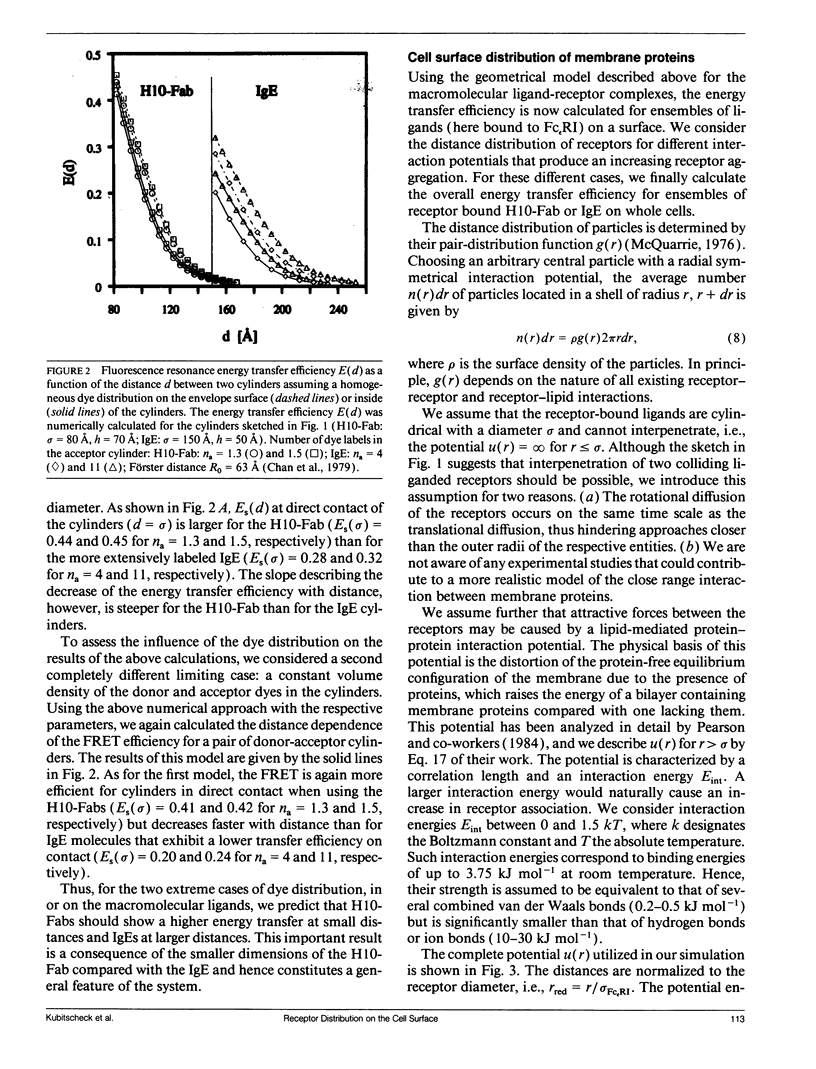
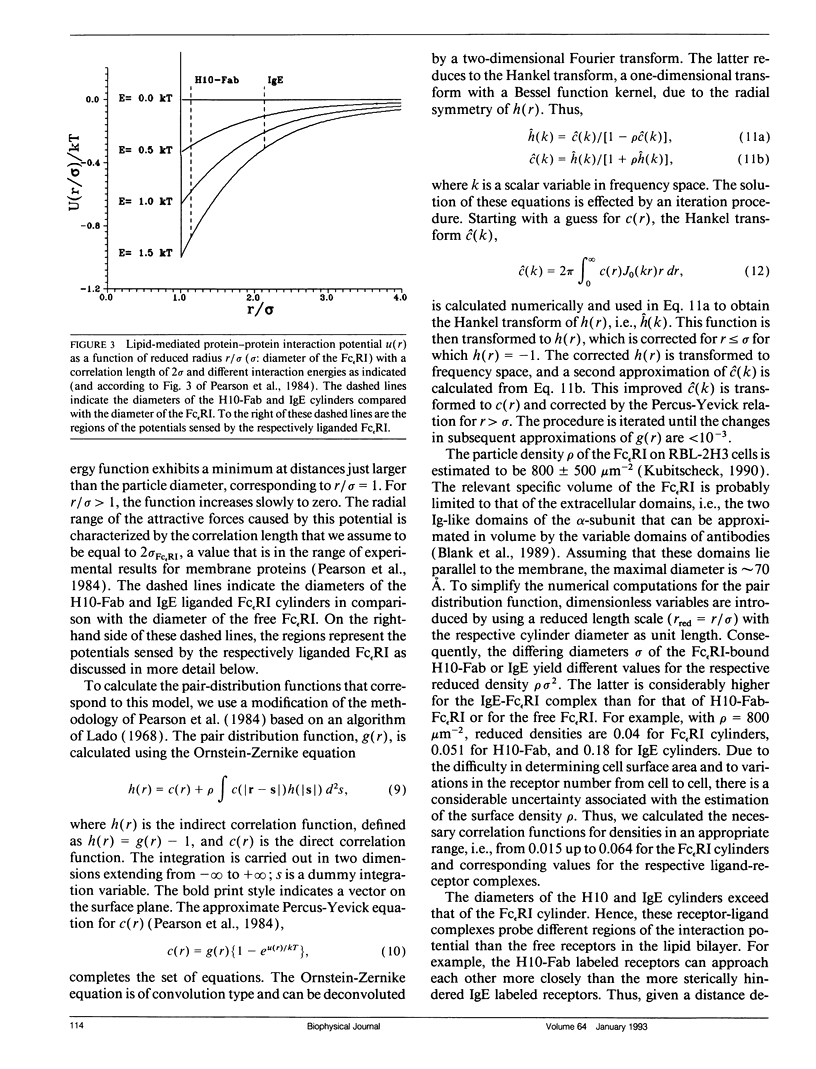
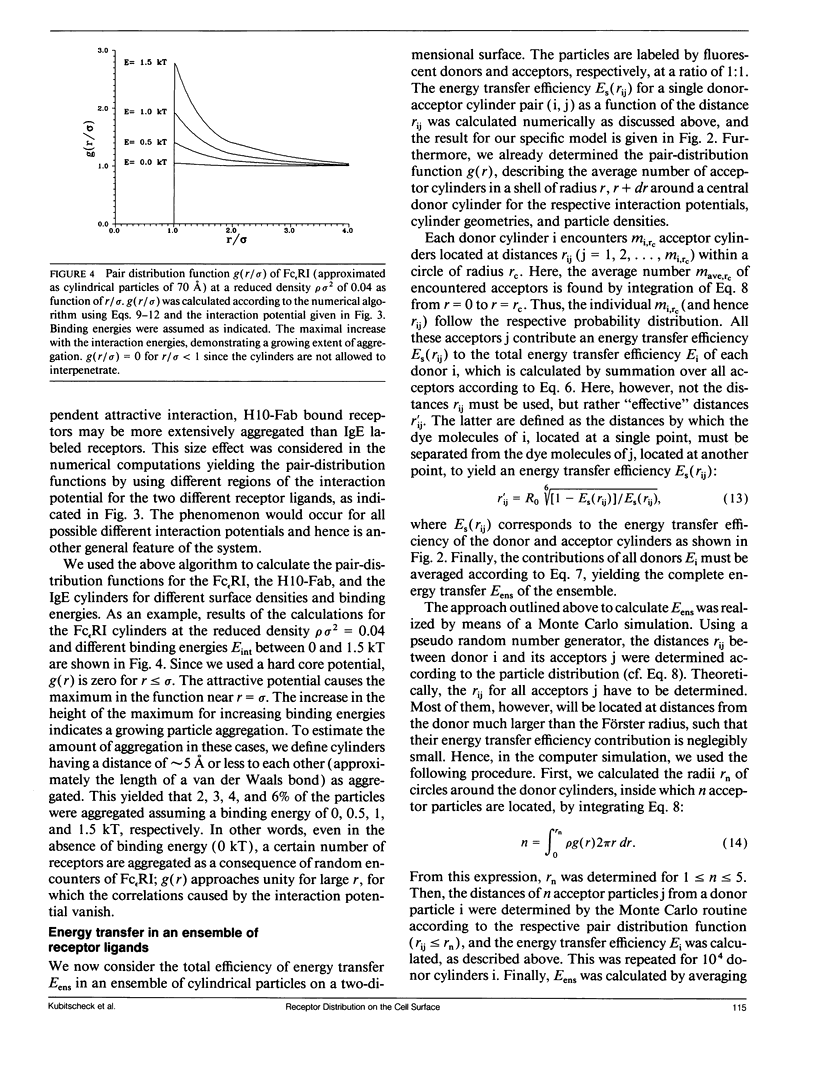
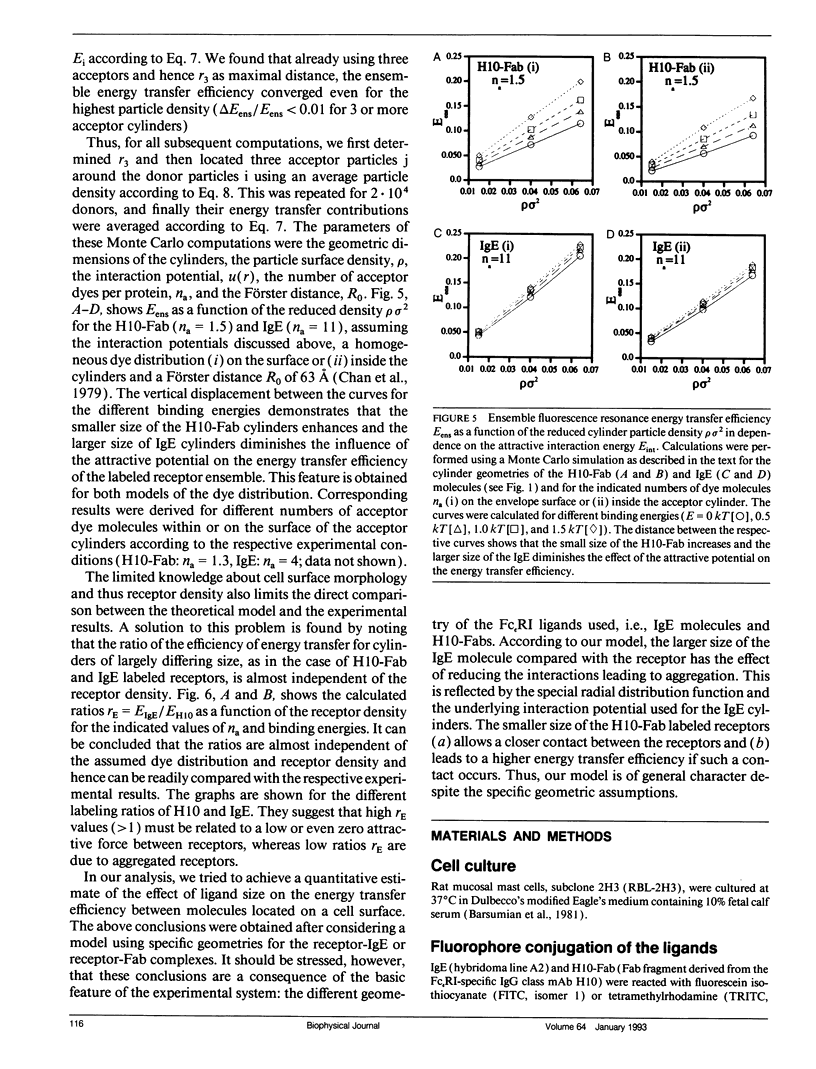
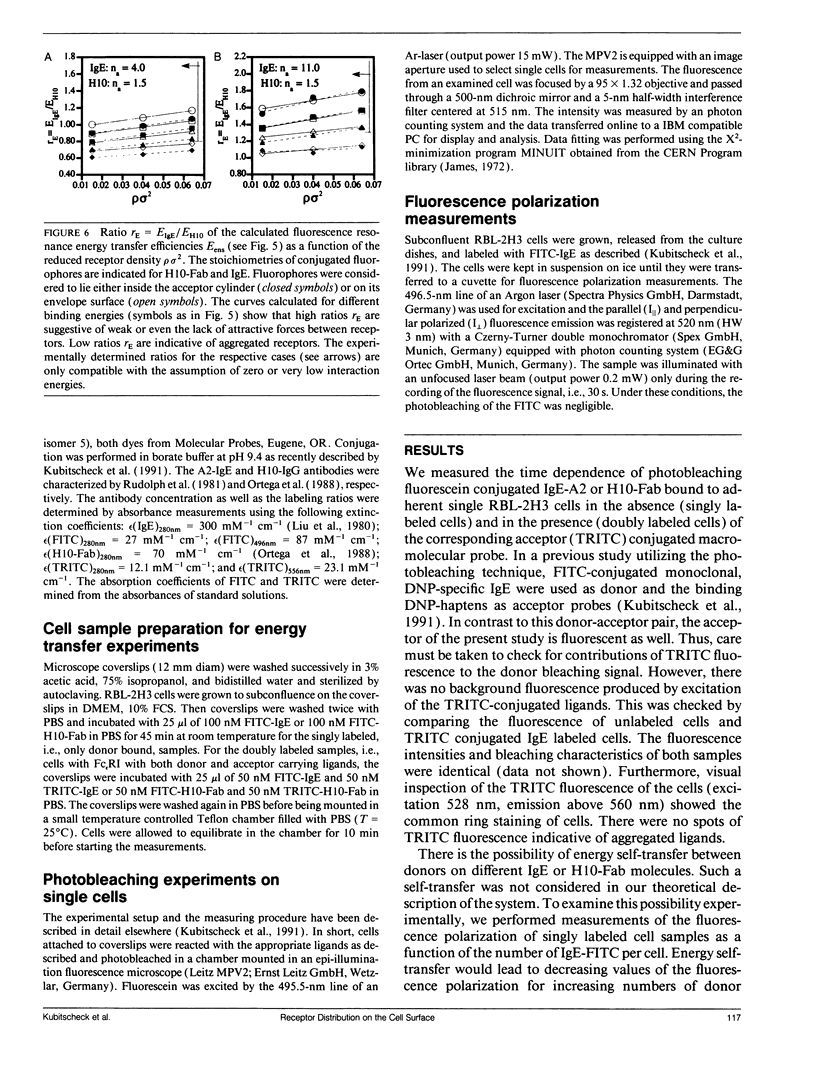
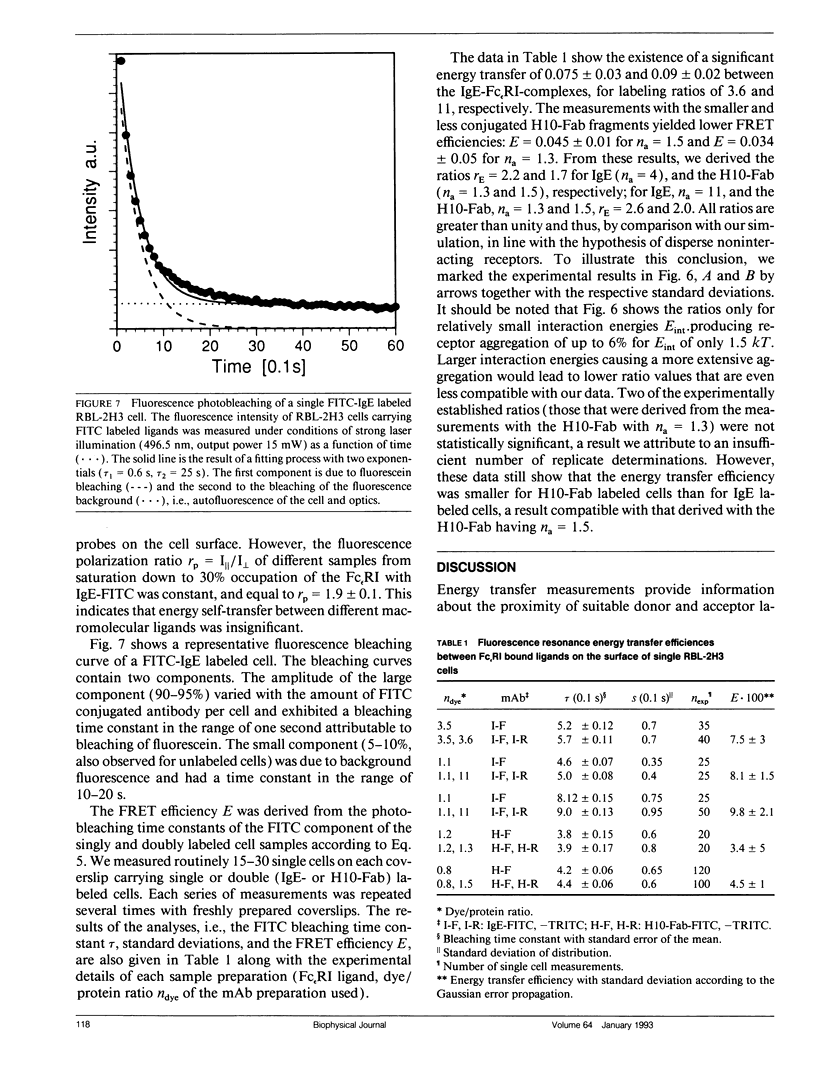
Abstract
The aggregation state of type I Fc epsilon-receptors (Fc epsilon RI) on the surface of single living mast cells was investigated by resonance fluorescence energy transfer. Derivatization of Fc epsilon RI specific ligands, i.e., immunoglobulin E or Fab fragments of a Fc epsilon RI specific monoclonal antibody, with donor and acceptor fluorophores provided a means for measuring receptor clustering through energy transfer between the receptor probes. The efficiency of energy transfer between the ligands carrying distinct fluorophores was determined on single cells in a microscope by analyzing the photobleaching kinetics of the donor fluorophore in the presence and absence of receptor ligands labeled with acceptor fluorophores. To rationalize the energy transfer data, we developed a theoretical model describing the dependence of the energy transfer efficiency on the geometry of the fluorescently labeled macromolecular ligands and their aggregation state on the cell surface. To this end, the transfer process was numerically calculated first for one pair and then for an ensemble of Fc epsilon RI bound ligands on the cell surface. The model stipulates that the aggregation state of the Fc epsilon RI is governed by an attractive lipid-protein mediated interaction potential. The corresponding pair-distribution function characterizes the spatial distribution of the ensemble. Using this approach, the energy transfer efficiency of the ensemble was calculated for different degrees of receptor aggregation. Comparison of the theoretical modeling results with the experimental energy transfer data clearly suggests that the Fc epsilon RI are monovalent, randomly distributed plasma membrane proteins. The method provides a novel approach for determining the aggregation state of cell surface components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Chan S. S., Arndt-Jovin D. J., Jovin T. M. Proximity of lectin receptors on the cell surface measured by fluorescence energy transfer in a flow system. J Histochem Cytochem. 1979 Jan;27(1):56–64. doi: 10.1177/27.1.374620. [DOI] [PubMed] [Google Scholar]
- Dewey T. G., Hammes G. G. Calculation on fluorescence resonance energy transfer on surfaces. Biophys J. 1980 Dec;32(3):1023–1035. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Posner R. G., Goldstein B., Holowka D., Baird B. Bivalent ligand dissociation kinetics from receptor-bound immunoglobulin E: evidence for a time-dependent increase in ligand rebinding at the cell surface. Biochemistry. 1991 Mar 5;30(9):2357–2363. doi: 10.1021/bi00223a009. [DOI] [PubMed] [Google Scholar]
- Erickson J., Kane P., Goldstein B., Holowka D., Baird B. Cross-linking of IgE-receptor complexes at the cell surface: a fluorescence method for studying the binding of monovalent and bivalent haptens to IgE. Mol Immunol. 1986 Jul;23(7):769–781. doi: 10.1016/0161-5890(86)90089-1. [DOI] [PubMed] [Google Scholar]
- Fagan M. H., Dewey T. G. Resonance energy transfer study of membrane-bound aggregates of the sarcoplasmic reticulum calcium ATPase. J Biol Chem. 1986 Mar 15;261(8):3654–3660. [PubMed] [Google Scholar]
- Gill G. N., Bertics P. J., Santon J. B. Epidermal growth factor and its receptor. Mol Cell Endocrinol. 1987 Jun;51(3):169–186. doi: 10.1016/0303-7207(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Holowka D., Conrad D. H., Baird B. Structural mapping of membrane-bound immunoglobulin E-receptor complexes: use of monoclonal anti-IgE antibodies to probe the conformation of receptor-bound IgE. Biochemistry. 1985 Oct 22;24(22):6260–6267. doi: 10.1021/bi00343a033. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Arndt-Jovin D. J. Luminescence digital imaging microscopy. Annu Rev Biophys Biophys Chem. 1989;18:271–308. doi: 10.1146/annurev.bb.18.060189.001415. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Holowka D., Baird B. Cross-linking of IgE-receptor complexes by rigid bivalent antigens greater than 200 A in length triggers cellular degranulation. J Cell Biol. 1988 Sep;107(3):969–980. doi: 10.1083/jcb.107.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck U., Kircheis M., Schweitzer-Stenner R., Dreybrodt W., Jovin T. M., Pecht I. Fluorescence resonance energy transfer on single living cells. Application to binding of monovalent haptens to cell-bound immunoglobulin E. Biophys J. 1991 Aug;60(2):307–318. doi: 10.1016/S0006-3495(91)82055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- McCloskey M. A., Liu Z. Y., Poo M. M. Lateral electromigration and diffusion of Fc epsilon receptors on rat basophilic leukemia cells: effects of IgE binding. J Cell Biol. 1984 Sep;99(3):778–787. doi: 10.1083/jcb.99.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza G., Metzger H. Distribution and valency of receptor for IgE on rodent mast cells and related tumour cells. Nature. 1976 Dec 9;264(5586):548–550. doi: 10.1038/264548a0. [DOI] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Myers J. N., Holowka D., Baird B. Rotational motion of monomeric and dimeric immunoglobulin E-receptor complexes. Biochemistry. 1992 Jan 21;31(2):567–575. doi: 10.1021/bi00117a038. [DOI] [PubMed] [Google Scholar]
- Ortega E., Schweitzer-Stenner R., Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988 Dec 20;7(13):4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. T., Chan S. I., Lewis B. A., Engelman D. M. Pair distribution functions of bacteriorhodopsin and rhodopsin in model bilayers. Biophys J. 1983 Aug;43(2):167–174. doi: 10.1016/S0006-3495(83)84337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. T., Edelman J., Chan S. I. Statistical mechanics of lipid membranes. Protein correlation functions and lipid ordering. Biophys J. 1984 May;45(5):863–871. doi: 10.1016/S0006-3495(84)84232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecht I., Ortega E., Jovin T. M. Rotational dynamics of the Fc epsilon receptor on mast cells monitored by specific monoclonal antibodies and IgE. Biochemistry. 1991 Apr 9;30(14):3450–3458. doi: 10.1021/bi00228a015. [DOI] [PubMed] [Google Scholar]
- Perelson A. S. Spatial distribution of surface immunoglobulin on B lymphocytes. Local ordering. Exp Cell Res. 1978 Mar 15;112(2):309–321. doi: 10.1016/0014-4827(78)90214-8. [DOI] [PubMed] [Google Scholar]
- Rudolph A. K., Burrows P. D., Wabl M. R. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981 Jun;11(6):527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Myers J., Holowka D., Baird B., Webb W. W. Molecular crowding on the cell surface. Science. 1988 Jan 1;239(4835):61–64. doi: 10.1126/science.2962287. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Licht A., Lüscher I., Pecht I. Oligomerization and ring closure of immunoglobulin E class antibodies by divalent haptens. Biochemistry. 1987 Jun 16;26(12):3602–3612. doi: 10.1021/bi00386a053. [DOI] [PubMed] [Google Scholar]
- Sims P. J. Complement protein C9 labeled with fluorescein isothiocyanate can be used to monitor C9 polymerization and formation of the cytolytic membrane lesion. Biochemistry. 1984 Jul 3;23(14):3248–3260. doi: 10.1021/bi00309a020. [DOI] [PubMed] [Google Scholar]
- Sullivan A. L., Grimley P. M., Metzger H. Electron microscopic localization of immunoglobulin E on the surface membrane of human basophils. J Exp Med. 1971 Dec 1;134(6):1403–1416. doi: 10.1084/jem.134.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Shopes B., Holowka D., Baird B. Dynamic conformations compared for IgE and IgG1 in solution and bound to receptors. Biochemistry. 1992 Aug 25;31(33):7446–7456. doi: 10.1021/bi00148a004. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Bartholdi M., Arndt-Jovin D., Jovin T. M. Rotational dynamics of the Fc receptor for immunoglobulin E on histamine-releasing rat basophilic leukemia cells. Biochemistry. 1986 Jul 29;25(15):4397–4401. doi: 10.1021/bi00363a033. [DOI] [PubMed] [Google Scholar]


