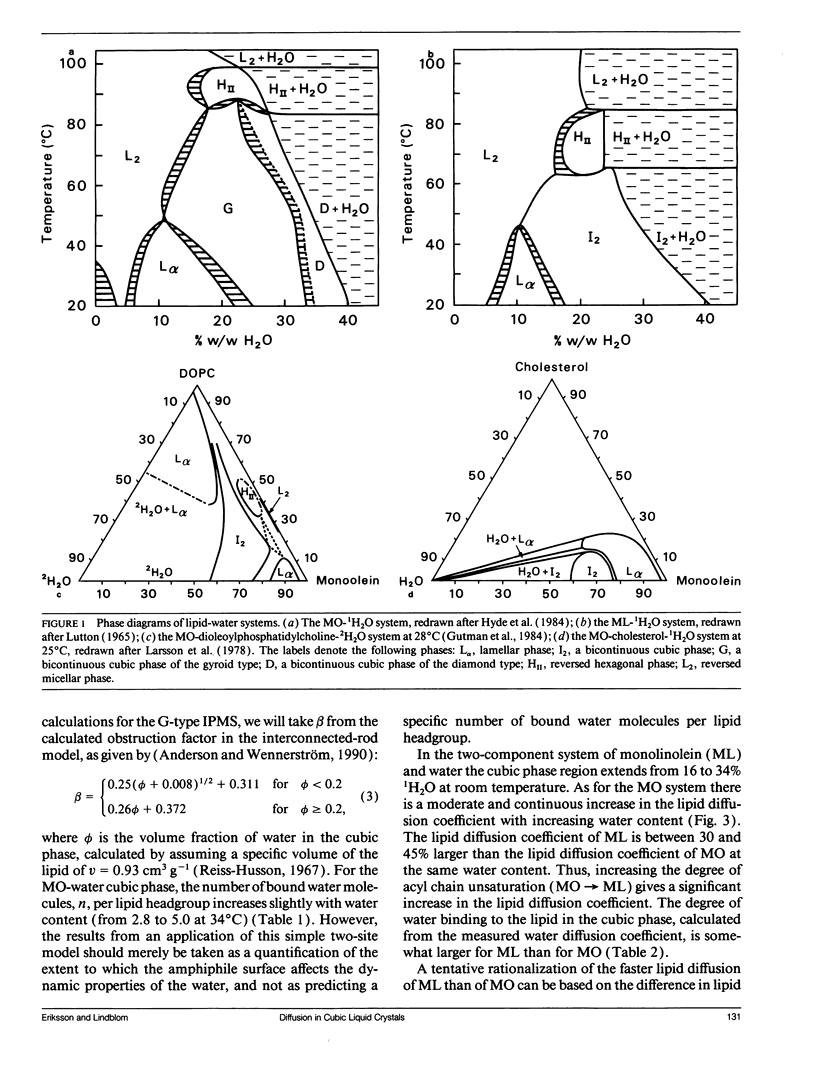
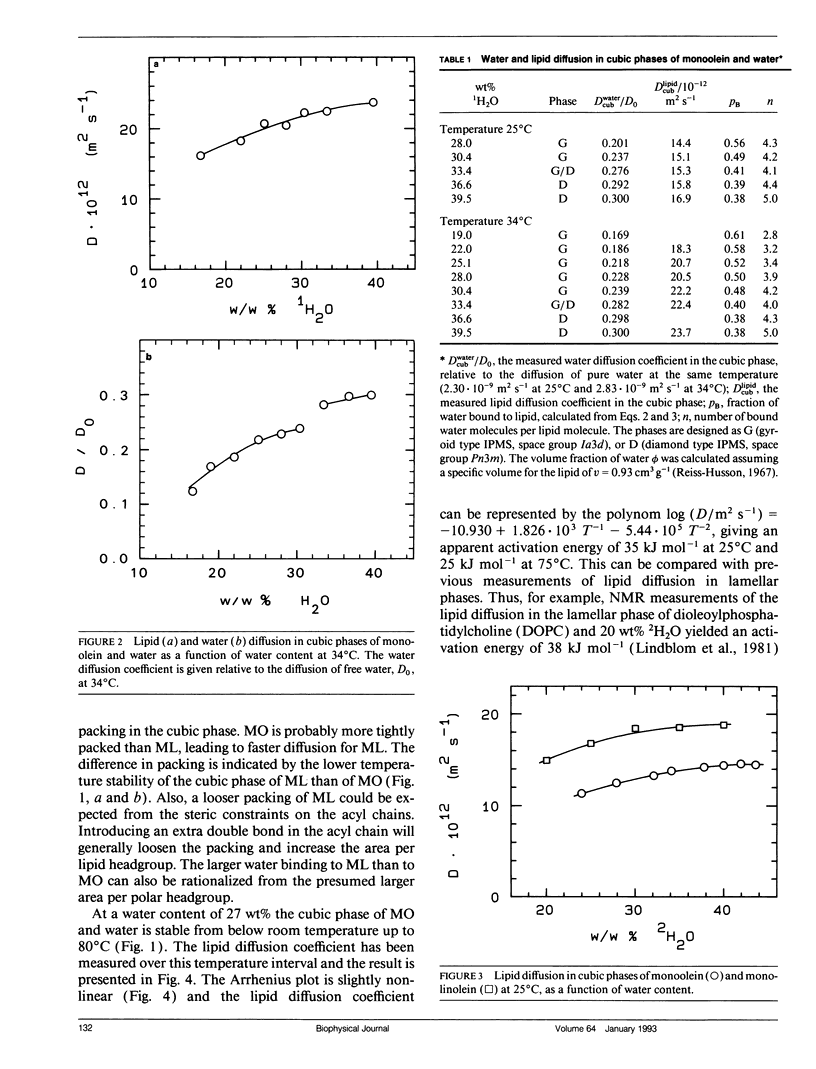
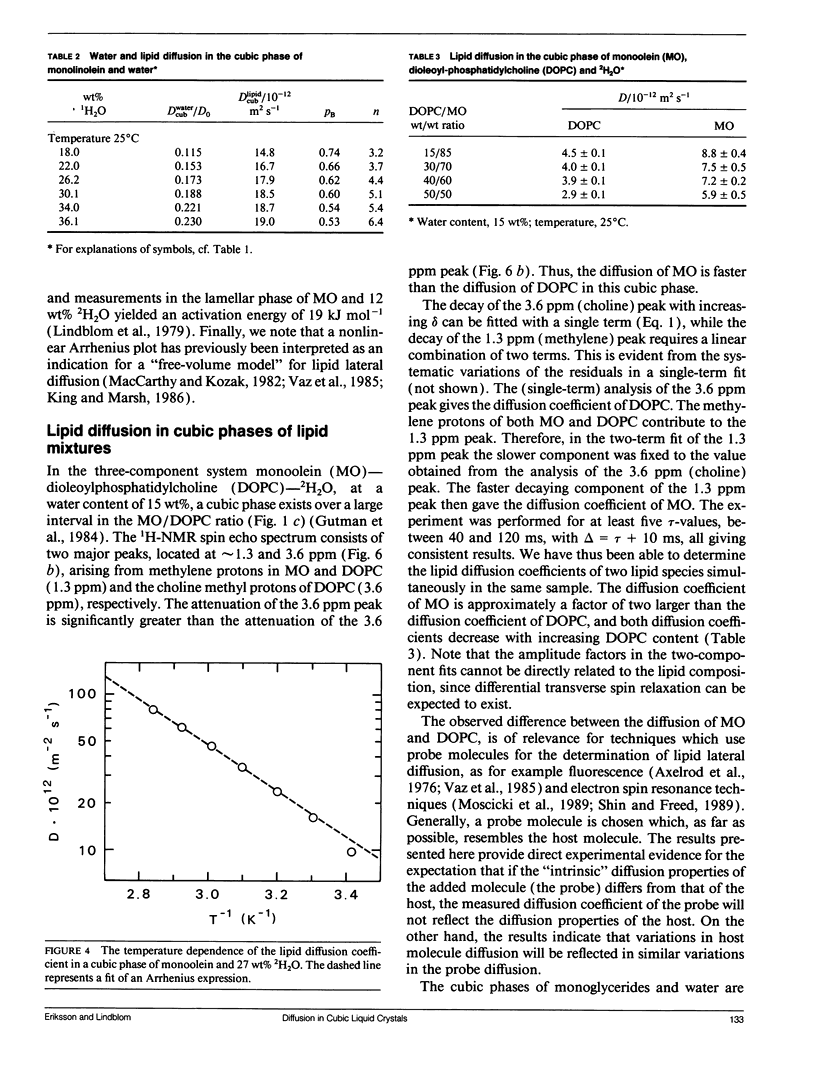
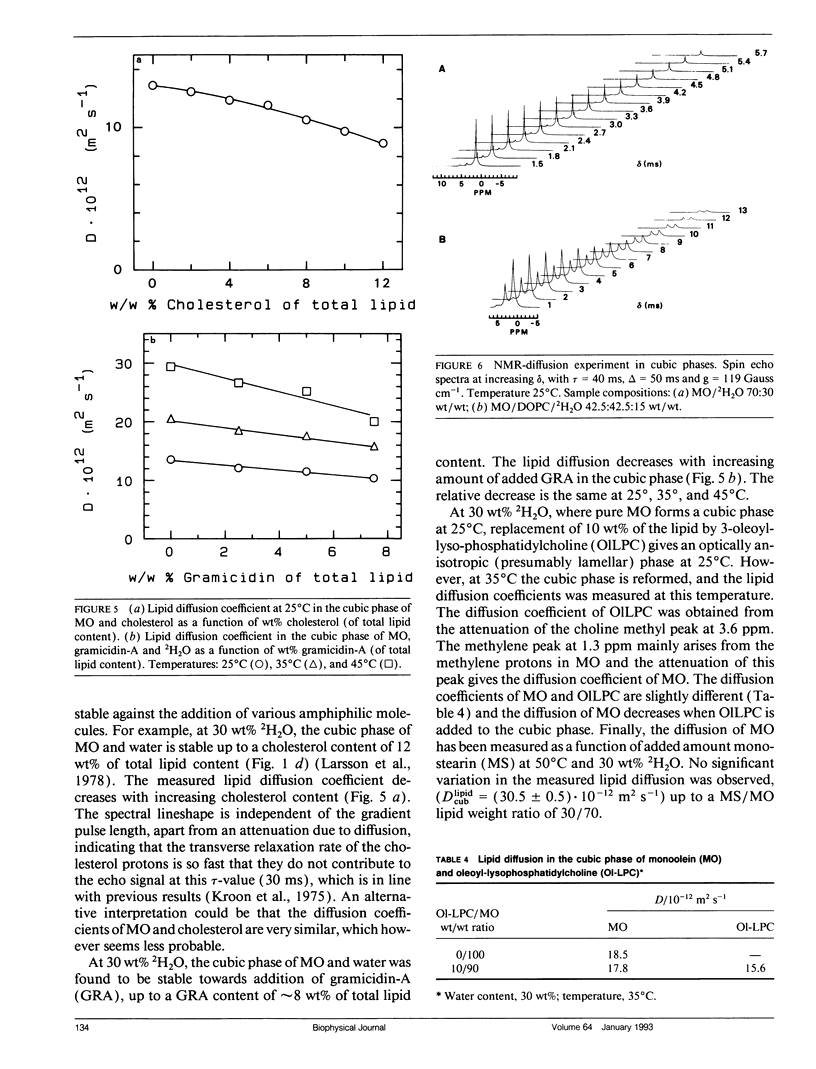
Abstract
Lipid and water diffusion coefficients in bicontinuous cubic liquid crystalline phases have been determined with the NMR pulsed magnetic field gradient technique. In the monoolein-water system, a discontinuity in the variation of the water diffusion coefficient with water content is observed, which coincides with the two-phase region between the two cubic phases in this system. The degree of water association to the lipid has been determined, considering the obstruction factor for diffusion in the cubic phases. The lipid diffusion coefficient increases with increased unsaturation of the lipid, and decreases when larger amphiphile molecules like cholesterol, gramicidin-A, and lyso-oleoyl-phosphatidylcholine are solubilized in the cubic phase. In a cubic liquid crystal of monoolein (MO), dioleoylphosphatidylcholine (DOPC), and water, the individual lipid diffusion coefficients have been determined simultaneously in the same sample. The diffusion coefficients of MO and DOPC differ by a factor of two, and both decrease with increasing DOPC content. The results are discussed in relation to probe techniques for measurements of lipid diffusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson B., Larsson K., Fontell K. A cubic protein-monoolein-water phase. Biochim Biophys Acta. 1983 Mar 23;729(1):23–27. doi: 10.1016/0005-2736(83)90451-0. [DOI] [PubMed] [Google Scholar]
- King M. D., Marsh D. Free volume model for lipid lateral diffusion coefficients. Assessment of the temperature dependence in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1986 Nov 6;862(1):231–234. doi: 10.1016/0005-2736(86)90489-x. [DOI] [PubMed] [Google Scholar]
- Kroon P. A., Kainosho M., Chan S. I. State of molecular motion of cholesterol in lecithin bilayers. Nature. 1975 Aug 14;256(5518):582–584. doi: 10.1038/256582a0. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Johansson L. B., Arvidson G. Effect of cholesterol in membranes. Pulsed nuclear magnetic resonance measurements of lipid lateral diffusion. Biochemistry. 1981 Apr 14;20(8):2204–2207. doi: 10.1021/bi00511a020. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Johansson L. B., Wikander G., Eriksson P. O., Arvidson G. Further evidence for closed, nonspherical aggregates in the cubic I(1) phase of lysolecithin and water. Biophys J. 1992 Sep;63(3):723–729. doi: 10.1016/s0006-3495(92)81642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom G., Wennerström H. Amphiphile diffusion in model membrane systems studied by pulsed NMR. Biophys Chem. 1977 Jan;6(2):167–171. doi: 10.1016/0301-4622(77)87006-3. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Wennerström H., Arvidson G., Lindman B. Lecithin translational diffusion studied by pulsed nuclear magnetic resonance. Biophys J. 1976 Nov;16(11):1287–1295. doi: 10.1016/S0006-3495(76)85774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutton E. S. Phase behavior of aqueous systems of monoglycerides. J Am Oil Chem Soc. 1965 Dec;42(12):1068–1070. doi: 10.1007/BF02636909. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Tardieu A., Gulik-Krzywicki T., Rivas E., Reiss-Husson F. Structure of the cubic phases of lipid-water systems. Nature. 1968 Nov 2;220(5166):485–488. doi: 10.1038/220485a0. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Rilfors L., Eriksson P. O., Arvidson G., Lindblom G. Relationship between three-dimensional arrays of "lipidic particles" and bicontinuous cubic lipid phases. Biochemistry. 1986 Nov 18;25(23):7702–7711. doi: 10.1021/bi00371a063. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. K., Freed J. H. Dynamic imaging of lateral diffusion by electron spin resonance and study of rotational dynamics in model membranes. Effect of cholesterol. Biophys J. 1989 Mar;55(3):537–550. doi: 10.1016/S0006-3495(89)82847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]


