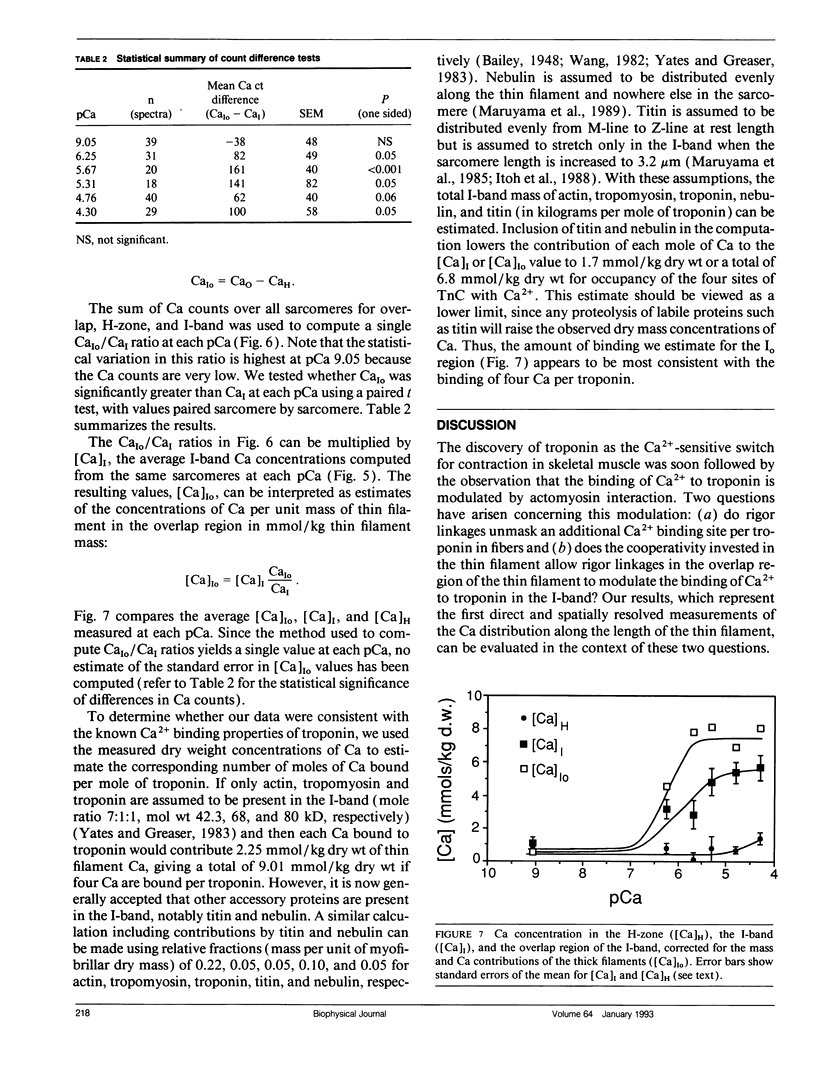
Abstract
Direct measurements were made of the Ca distribution within sarcomeres of glycerinated rabbit psoas muscle fibers in rigor using electron probe x-ray microanalysis. Both analogue raster analysis and digital x-ray imaging were used to quantitate the Ca distribution along thick and thin filaments as a function of the concentration of free Ca2+. Even when corrected for the estimated contribution of Ca bound to thick filaments, the Ca measured in the region of overlap between thick and thin filaments significantly exceeded the Ca in the I-band at subsaturating concentrations of free Ca2+. At saturating levels of free Ca2+, the excess Ca in the overlap region was diminished but still statistically significant. The data thus suggest that the formation of rigor linkages exerts multiple effects on the binding of Ca2+ to thin filaments in the overlap region by increasing the affinity of troponin C for Ca2+ and possibly by unmasking additional Ca2+ binding sites. The data also show that the cooperativity invested in the thin filaments is insufficient to permit the effects of rigor cross-bridge formation on Ca2+ binding to propagate far along the thin filaments into the I-band.
Full text
PDF
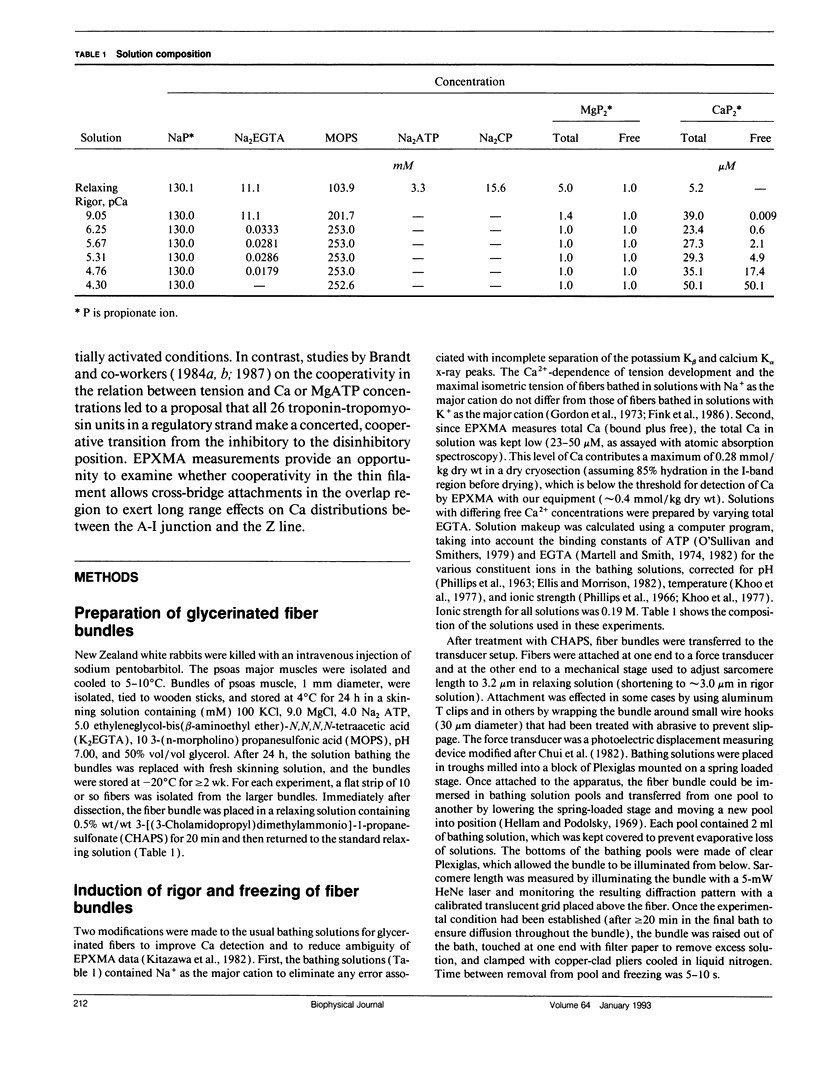


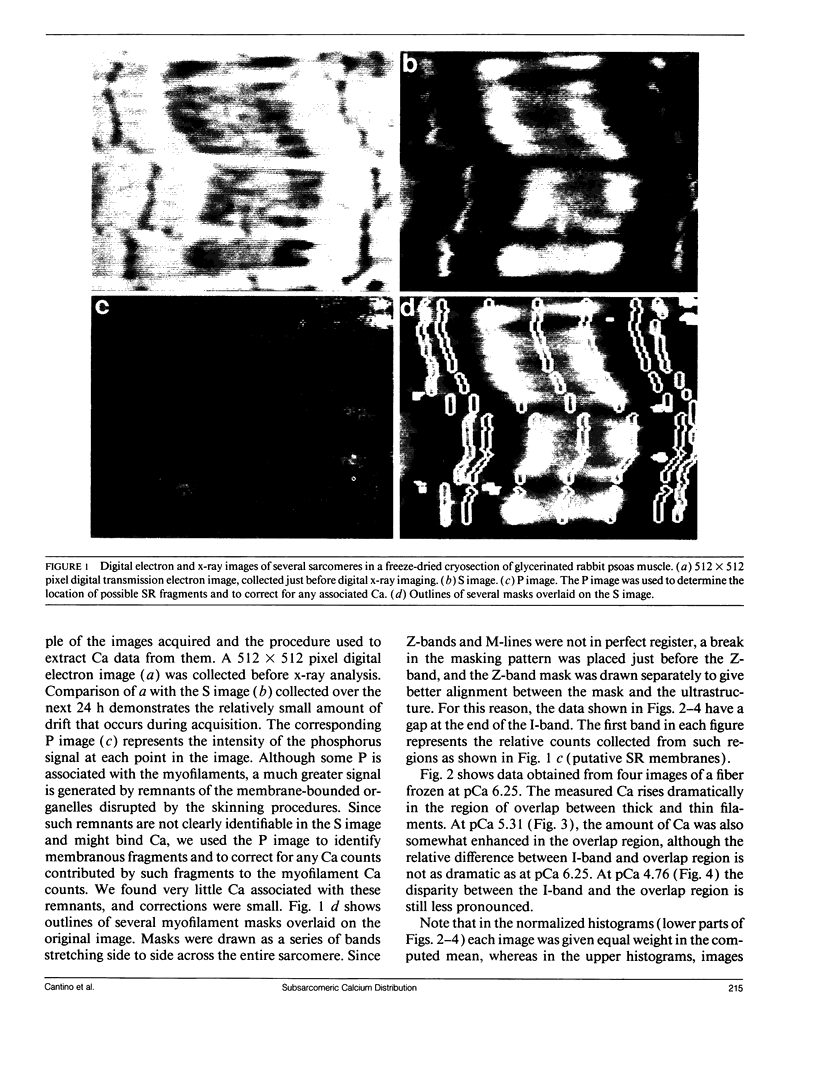
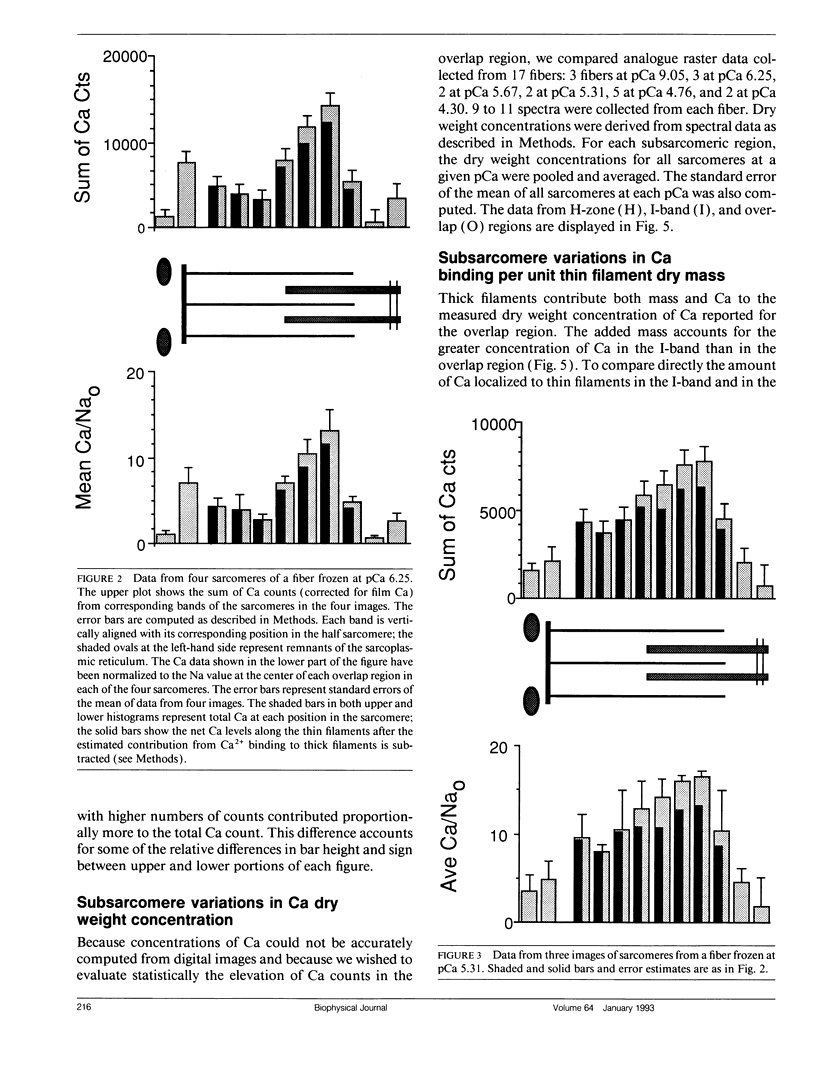
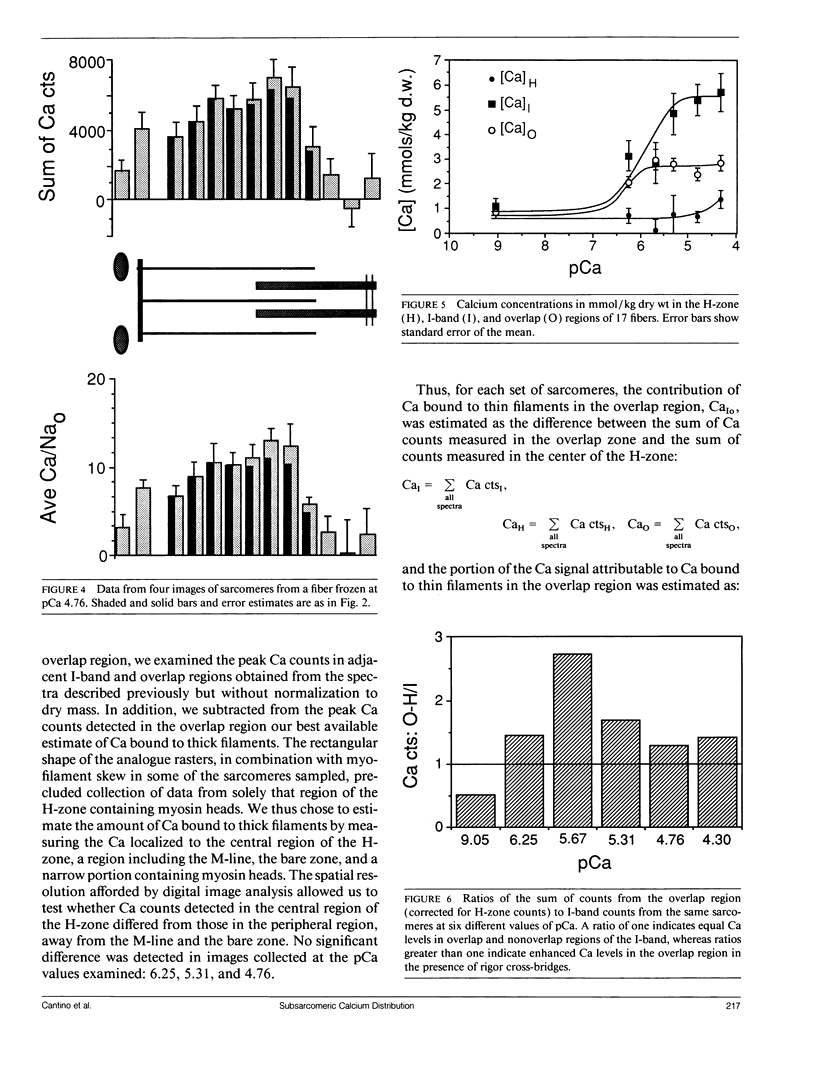




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. S., Yates L. D., Gordon A. M. Ca(2+)-dependence of structural changes in troponin-C in demembranated fibers of rabbit psoas muscle. Biophys J. 1992 Feb;61(2):399–409. doi: 10.1016/S0006-3495(92)81846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Rutchik J. S., Schachat F. H. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987 Jun 20;195(4):885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Roemer D., Schachat F. H. Co-operative activation of skeletal muscle thin filaments by rigor crossbridges. The effect of troponin C extraction. J Mol Biol. 1990 Apr 5;212(3):473–480. doi: 10.1016/0022-2836(90)90326-H. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Cantino M. E., Wilkinson L. E., Goddard M. K., Johnson D. E. Beam induced mass loss in high resolution biological microanalysis. J Microsc. 1986 Dec;144(Pt 3):317–327. doi: 10.1111/j.1365-2818.1986.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Chiu Y. L., Asayama J., Ford L. E. A sensitive photoelectric force transducer with a resonant frequency of 6 kHz. Am J Physiol. 1982 Nov;243(5):C299–C302. doi: 10.1152/ajpcell.1982.243.5.C299. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ellis K. J., Morrison J. F. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986 Jan;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F., Black B. The effect of magnesium ions on the binding of calcium ions to glycerinated rabbit psoas muscle fibers. Biochim Biophys Acta. 1980 Mar 26;622(1):52–62. doi: 10.1016/0005-2795(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Fuchs F. Cooperative interactions between calcium-binding sites on glycerinated muscle fibers. The influence of cross-bridge attachment. Biochim Biophys Acta. 1977 Nov 17;462(2):314–322. doi: 10.1016/0005-2728(77)90130-x. [DOI] [PubMed] [Google Scholar]
- Fuchs F. On the relation between filament overlap and the number of calcium-binding sites on glycerinated muscle fibers. Biophys J. 1978 Mar;21(3):273–277. doi: 10.1016/S0006-3495(78)85524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F. The binding of calcium to detergent-extracted rabbit psoas muscle fibres during relaxation and force generation. J Muscle Res Cell Motil. 1985 Aug;6(4):477–486. doi: 10.1007/BF00712584. [DOI] [PubMed] [Google Scholar]
- Fuchs F. The binding of calcium to glycerinated muscle fibers in rigor. The effect of filament overlap. Biochim Biophys Acta. 1977 Apr 25;491(2):523–531. doi: 10.1016/0005-2795(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Fuchs F., Wang Y. P. Force, length, and Ca(2+)-troponin C affinity in skeletal muscle. Am J Physiol. 1991 Nov;261(5 Pt 1):C787–C792. doi: 10.1152/ajpcell.1991.261.5.C787. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B., Yates L. D., Allen T. Muscle cross-bridge attachment: effects on calcium binding and calcium activation. Adv Exp Med Biol. 1988;226:89–99. [PubMed] [Google Scholar]
- Grabarek Z., Grabarek J., Leavis P. C., Gergely J. Cooperative binding to the Ca2+-specific sites of troponin C in regulated actin and actomyosin. J Biol Chem. 1983 Dec 10;258(23):14098–14102. [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hall T. A., Gupta B. L. Quantification for the x-ray microanalysis of cryosections. J Microsc. 1982 Jun;126(Pt 3):333–345. doi: 10.1111/j.1365-2818.1982.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol. 1987 Jul;253(1 Pt 1):C90–C96. doi: 10.1152/ajpcell.1987.253.1.C90. [DOI] [PubMed] [Google Scholar]
- Hofmann P. A., Metzger J. M., Greaser M. L., Moss R. L. Effects of partial extraction of light chain 2 on the Ca2+ sensitivities of isometric tension, stiffness, and velocity of shortening in skinned skeletal muscle fibers. J Gen Physiol. 1990 Mar;95(3):477–498. doi: 10.1085/jgp.95.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Suzuki T., Kimura S., Ohashi K., Higuchi H., Sawada H., Shimizu T., Shibata M., Maruyama K. Extensible and less-extensible domains of connectin filaments in stretched vertebrate skeletal muscle sarcomeres as detected by immunofluorescence and immunoelectron microscopy using monoclonal antibodies. J Biochem. 1988 Oct;104(4):504–508. doi: 10.1093/oxfordjournals.jbchem.a122499. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Shuman H., Somlyo A. P. Calcium and magnesium binding to thin and thick filaments in skinned muscle fibres: electron probe analysis. J Muscle Res Cell Motil. 1982 Dec;3(4):437–454. doi: 10.1007/BF00712093. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Matsuno A., Higuchi H., Shimaoka S., Kimura S., Shimizu T. Behaviour of connectin (titin) and nebulin in skinned muscle fibres released after extreme stretch as revealed by immunoelectron microscopy. J Muscle Res Cell Motil. 1989 Oct;10(5):350–359. doi: 10.1007/BF01758431. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Yoshioka T., Higuchi H., Ohashi K., Kimura S., Natori R. Connectin filaments link thick filaments and Z lines in frog skeletal muscle as revealed by immunoelectron microscopy. J Cell Biol. 1985 Dec;101(6):2167–2172. doi: 10.1083/jcb.101.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan W. J., Smithers G. W. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- PHILLIPS R. C., GEORGE P., RUTMAN R. J. POTENTIOMETRIC STUDIES OF THE SECONDARY PHOSPHATE IONIZATIONS OF AMP, ADP, AND ATP, AND CALCULATIONS OF THERMODYNAMIC DATA FOR THE HYDROLYSIS REACTIONS. Biochemistry. 1963 May-Jun;2:501–508. doi: 10.1021/bi00903a019. [DOI] [PubMed] [Google Scholar]
- Pan B. S., Solaro R. J. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987 Jun 5;262(16):7839–7849. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M. Muscle calcium transient. Effect of post-stimulus length changes in single fibers. J Gen Physiol. 1984 Jan;83(1):75–103. doi: 10.1085/jgp.83.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium and magnesium binding to troponin C. J Biol Chem. 1985 Jan 10;260(1):242–251. [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Electron probe X-ray microanalysis of Ca2+, Mg2+, and other ions in rapidly frozen cells. Methods Enzymol. 1989;172:203–229. doi: 10.1016/s0076-6879(89)72016-4. [DOI] [PubMed] [Google Scholar]
- Swartz D. R., Greaser M. L., Marsh B. B. Regulation of binding of subfragment 1 in isolated rigor myofibrils. J Cell Biol. 1990 Dec;111(6 Pt 2):2989–3001. doi: 10.1083/jcb.111.6.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman L. S., Sawyer D. Calcium binds cooperatively to the regulatory sites of the cardiac thin filament. J Biol Chem. 1990 Jan 15;265(2):931–939. [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Purification of titin and nebulin. Methods Enzymol. 1982;85(Pt B):264–274. doi: 10.1016/0076-6879(82)85025-8. [DOI] [PubMed] [Google Scholar]
- Wegner A., Walsh T. P. Interaction of tropomyosin-troponin with actin filaments. Biochemistry. 1981 Sep 15;20(19):5633–5642. doi: 10.1021/bi00522a043. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]



