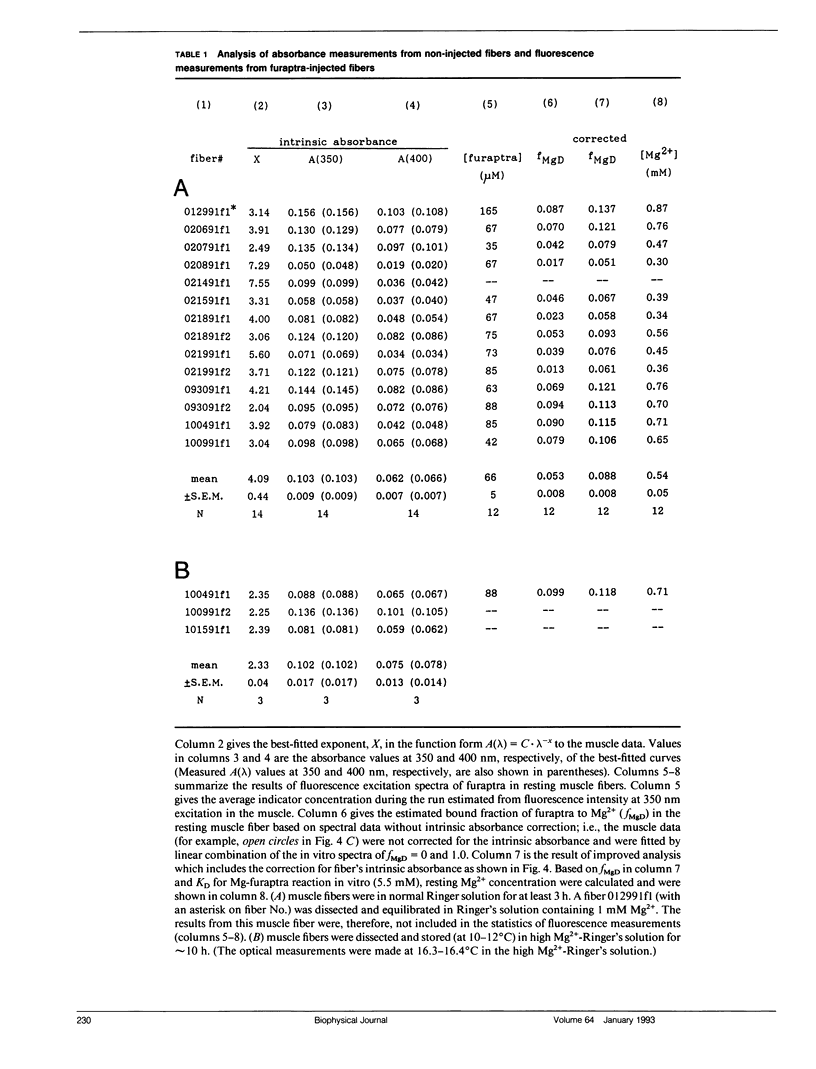
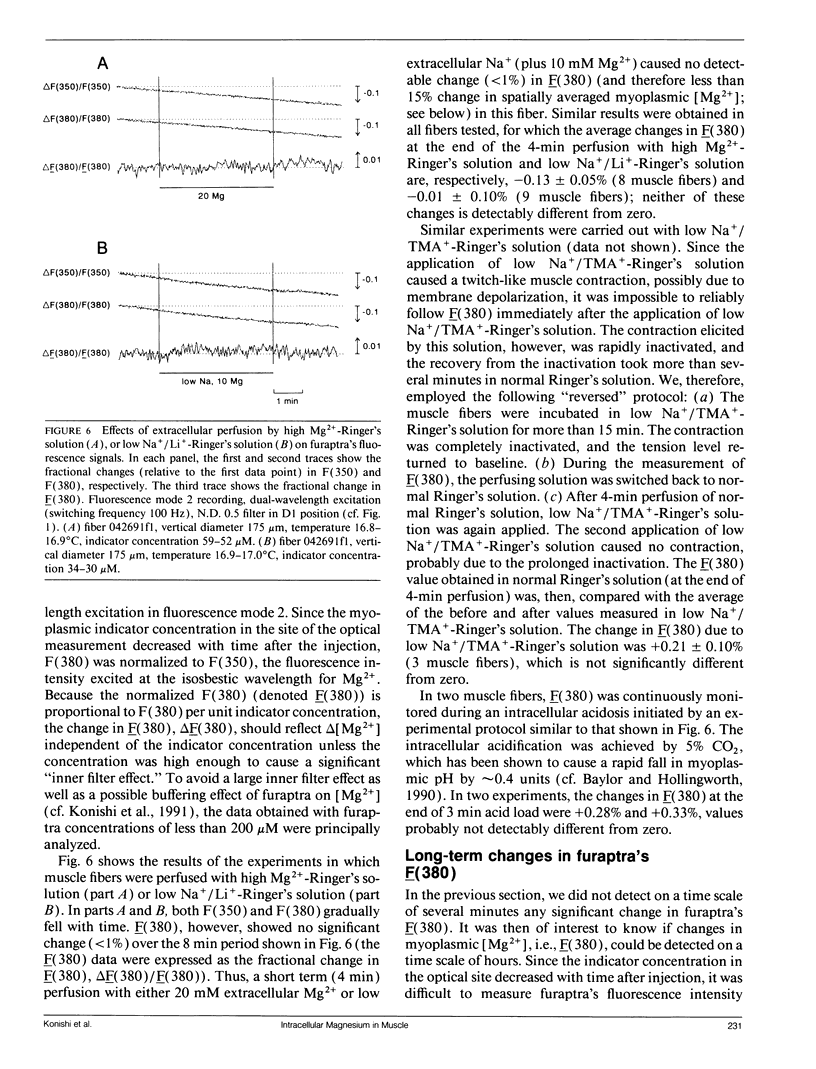
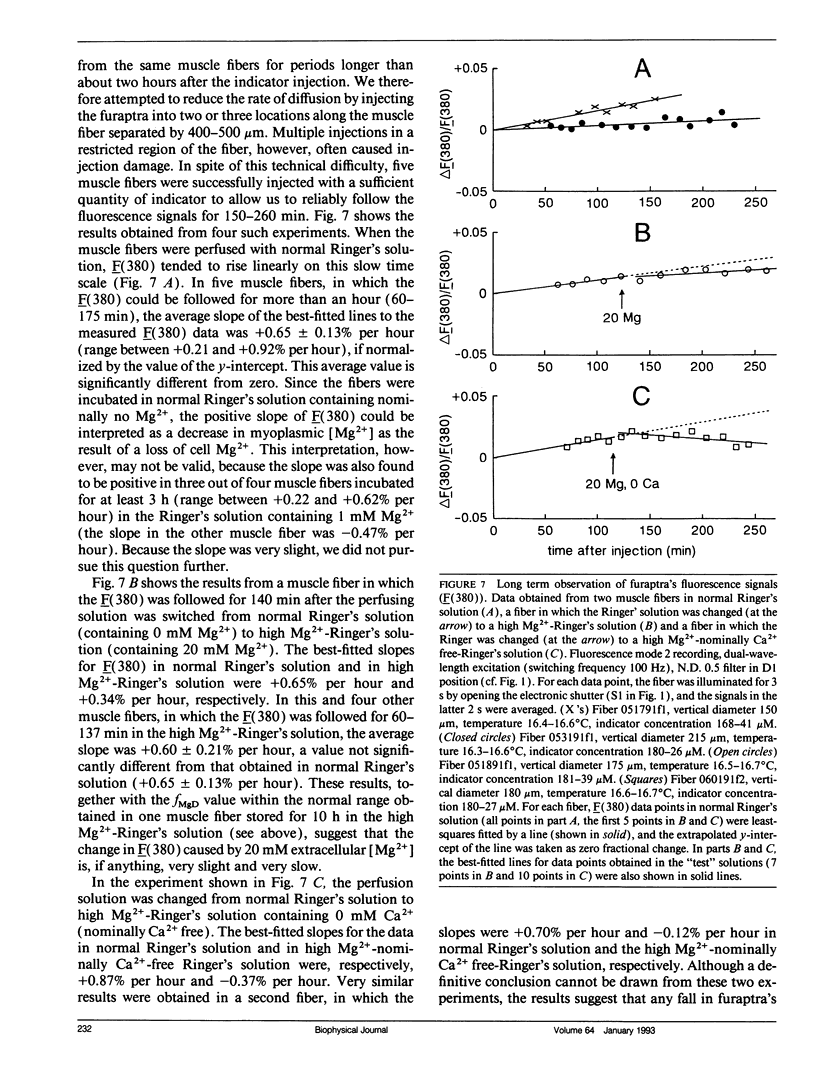
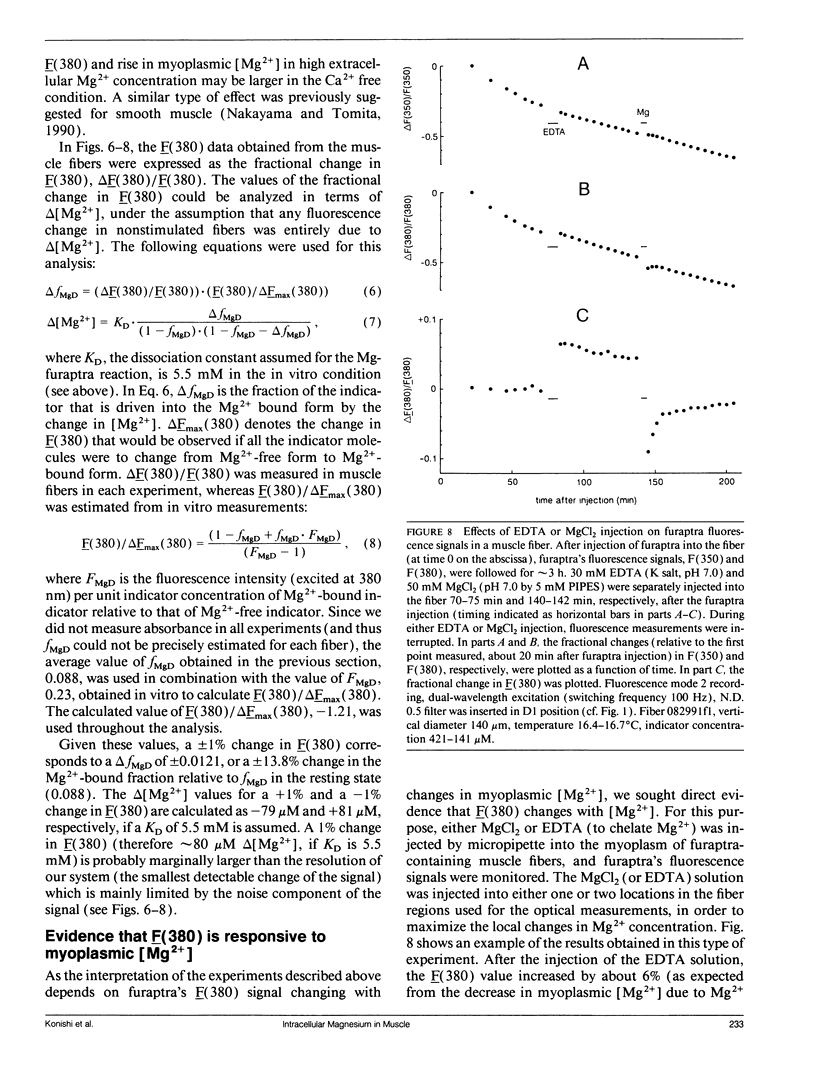
Abstract
The fluorescent Mg2+/Ca2+ indicator, furaptra, was injected into single frog skeletal muscle fibers, and the indicator's fluorescence signals were measured and analyzed with particular interest in the free Mg2+ concentration ([Mg2+]) in resting muscle. Based on the fluorescence excitation spectrum of furaptra, the calibrated myoplasmic [Mg2+] level averaged 0.54 mM, if the value of dissociation constant (KD) for Mg2+ obtained in vitro (5.5 mM) was used. However, if the indicator reacts with Mg2+ with a two-fold larger KD in myoplasm, as previously suggested for the furaptra-Ca2+ reaction (M. Konishi, S. Hollingworth, A.B. Harkins, S.M. Baylor. 1991. J. Gen. Physiol. 97:271-301), the calculated [Mg2+] would average 1.1 mM. Thus, the value 1.1 mM probably represents the best estimate from furaptra of [Mg2+] in resting muscle fibers. Extracellular perfusion of muscle fibers with high Mg2+ concentration solution or low Na+ concentration solution did not cause any detectable changes in the [Mg2+]-related furaptra fluorescence within 4 min. The results suggest that the myoplasmic [Mg2+] is highly regulated near the resting level of 1 mM, and that changes only occur with a very slow time course.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., González-Serratos H. Intracellular free magnesium in frog skeletal muscle fibres measured with ion-selective micro-electrodes. J Physiol. 1986 Sep;378:461–483. doi: 10.1113/jphysiol.1986.sp016230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C. The efflux of magnesium from single crustacean muscle fibres. J Physiol. 1972 Nov;226(3):653–674. doi: 10.1113/jphysiol.1972.sp010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Absorbance signals from resting frog skeletal muscle fibers injected with the pH indicator dye, phenol red. J Gen Physiol. 1990 Sep;96(3):449–471. doi: 10.1085/jgp.96.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Hui C. S., Quinta-Ferreira M. E. Properties of the metallochromic dyes Arsenazo III, Antipyrylazo III and Azo1 in frog skeletal muscle fibres at rest. J Physiol. 1986 Aug;377:89–141. doi: 10.1113/jphysiol.1986.sp016178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., Blinks J. R. Simultaneous measurement of Ca2+ in muscle with Ca electrodes and aequorin. Diffusible cytoplasmic constituent reduces Ca(2+)-independent luminescence of aequorin. J Gen Physiol. 1991 Dec;98(6):1141–1160. doi: 10.1085/jgp.98.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A. Intracellular free magnesium in frog skeletal muscle studied with a new type of magnesium-selective microelectrode: interactions between magnesium and sodium in the regulation of [Mg]i. Pflugers Arch. 1990 May;416(3):238–246. doi: 10.1007/BF00392059. [DOI] [PubMed] [Google Scholar]
- Buri A., McGuigan J. A. Intracellular free magnesium and its regulation, studied in isolated ferret ventricular muscle with ion-selective microelectrodes. Exp Physiol. 1990 Nov;75(6):751–761. doi: 10.1113/expphysiol.1990.sp003457. [DOI] [PubMed] [Google Scholar]
- Close R. I., Lännergren J. I. Arsenazo III calcium transients and latency relaxation in frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1984 Oct;355:323–344. doi: 10.1113/jphysiol.1984.sp015422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Garfinkel L., Garfinkel D. Calculation of free-Mg2+ concentration in adenosine 5'-triphosphate containing solutions in vitro and in vivo. Biochemistry. 1984 Jul 17;23(15):3547–3552. doi: 10.1021/bi00310a025. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol. 1988 May;254(5 Pt 1):C591–C604. doi: 10.1152/ajpcell.1988.254.5.C591. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Moore R. D. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G. Mechanism of calcium transport. Annu Rev Physiol. 1985;47:573–601. doi: 10.1146/annurev.ph.47.030185.003041. [DOI] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Simon B. J., Szucs G., Schneider M. F. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophys J. 1988 Jun;53(6):971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Walton C., Ling M. R. Mg++ and K+ distribution in frog muscle and egg: a disproof of the Donnan theory of membrane equilibrium applied to the living cells. J Cell Physiol. 1979 Nov;101(2):261–278. doi: 10.1002/jcp.1041010208. [DOI] [PubMed] [Google Scholar]
- London R. E. Methods for measurement of intracellular magnesium: NMR and fluorescence. Annu Rev Physiol. 1991;53:241–258. doi: 10.1146/annurev.ph.53.030191.001325. [DOI] [PubMed] [Google Scholar]
- López J. R., Alamo L., Caputo C., Vergara J., DiPolo R. Direct measurement of intracellular free magnesium in frog skeletal muscle using magnesium-selective microelectrodes. Biochim Biophys Acta. 1984 May 22;804(1):1–7. doi: 10.1016/0167-4889(84)90091-0. [DOI] [PubMed] [Google Scholar]
- Murphy E., Freudenrich C. C., Levy L. A., London R. E., Lieberman M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator Furaptra. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2981–2984. doi: 10.1073/pnas.86.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Regulation of intracellular free magnesium concentration in the taenia of guinea-pig caecum. J Physiol. 1991 Apr;435:559–572. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J. M., Kovács T. Functional and ionic changes accompanying magnesium penetration in skeletal muscle. Pflugers Arch. 1974;350(4):321–334. doi: 10.1007/BF00592640. [DOI] [PubMed] [Google Scholar]
- Popov E. G., Gavrilov Y. Iu, Pozin EYa, Gabbasov Z. A. Multiwavelength method for measuring concentration of free cytosolic calcium using the fluorescent probe indo-1. Arch Biochem Biophys. 1988 Feb 15;261(1):91–96. doi: 10.1016/0003-9861(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Suda N., Kurihara S. Intracellular calcium signals measured with fura-2 and aequorin in frog skeletal muscle fibers. Jpn J Physiol. 1991;41(2):277–295. doi: 10.2170/jjphysiol.41.277. [DOI] [PubMed] [Google Scholar]
- Uto A., Arai H., Ogawa Y. Reassessment of Fura-2 and the ratio method for determination of intracellular Ca2+ concentrations. Cell Calcium. 1991 Jan;12(1):29–37. doi: 10.1016/0143-4160(91)90082-p. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lamb A. G., Bolsover S. R., Allen D. G. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflugers Arch. 1990 Mar;415(6):734–740. doi: 10.1007/BF02584013. [DOI] [PubMed] [Google Scholar]


