Abstract
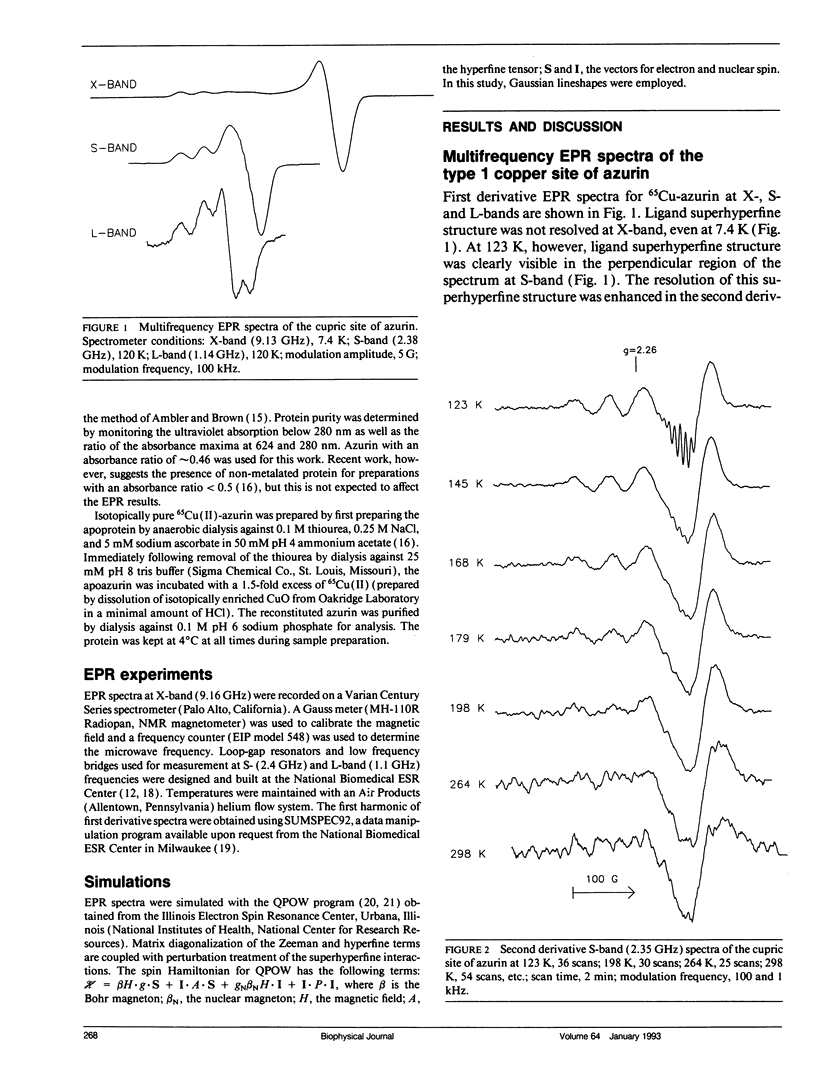
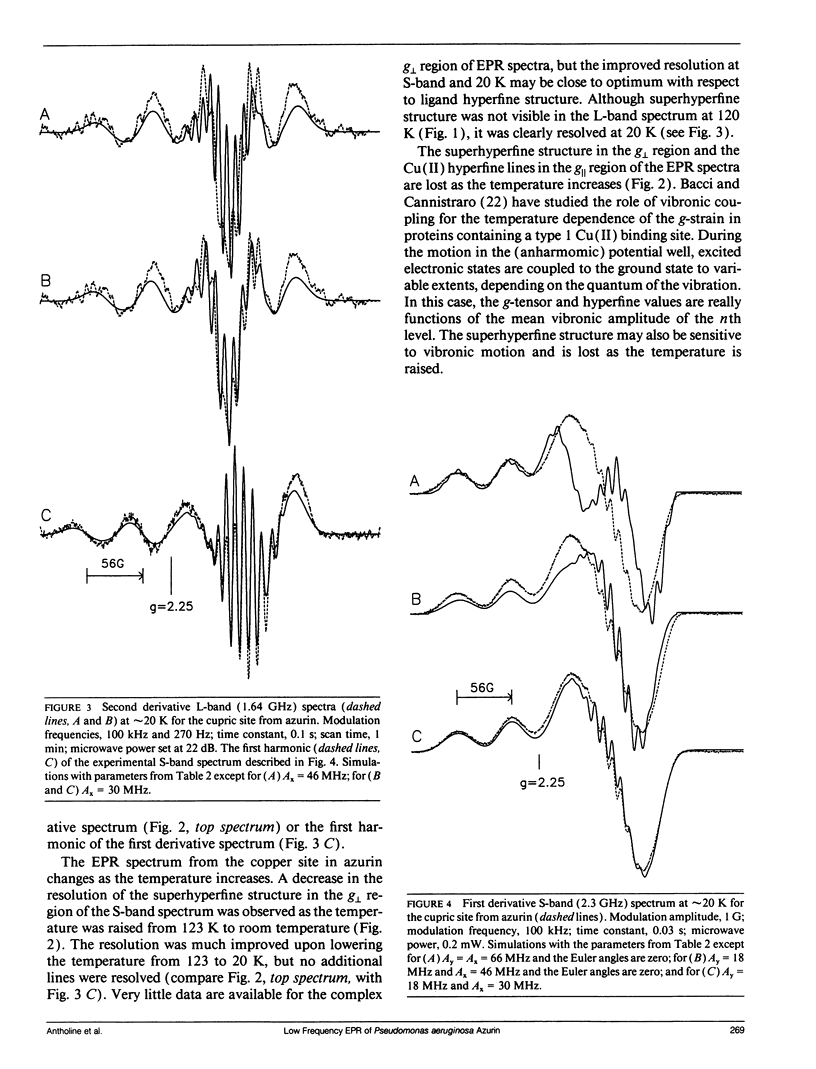
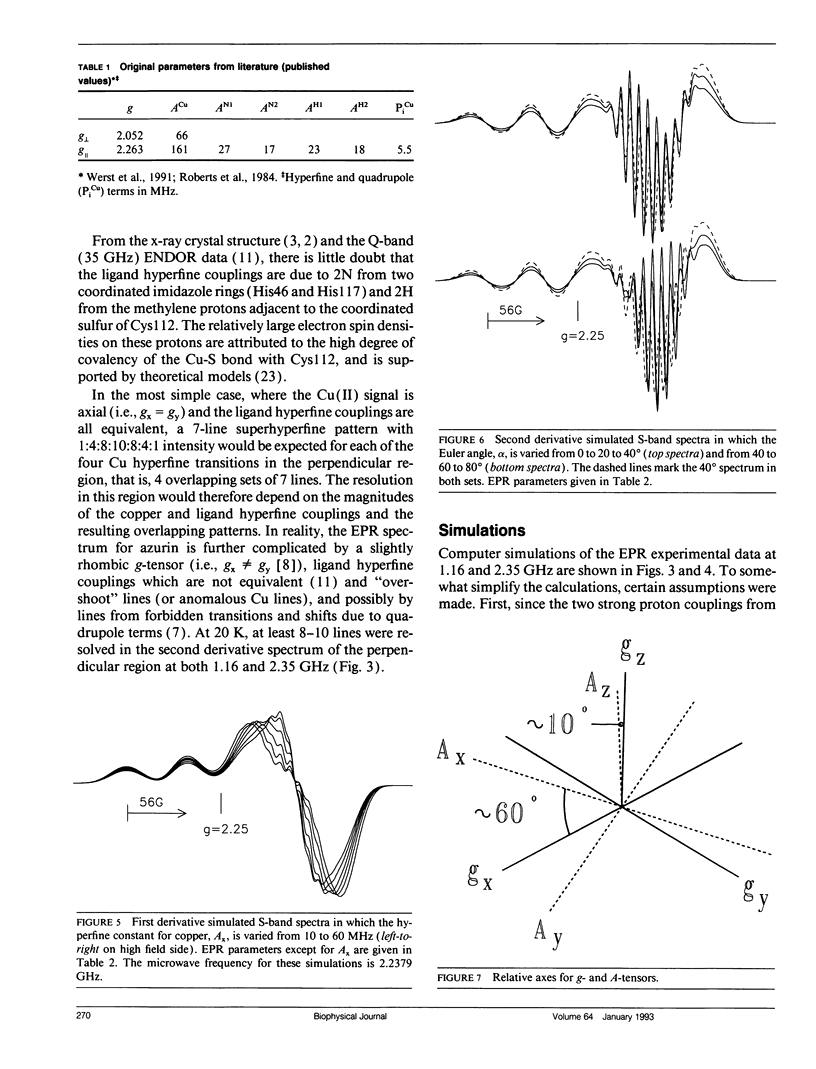
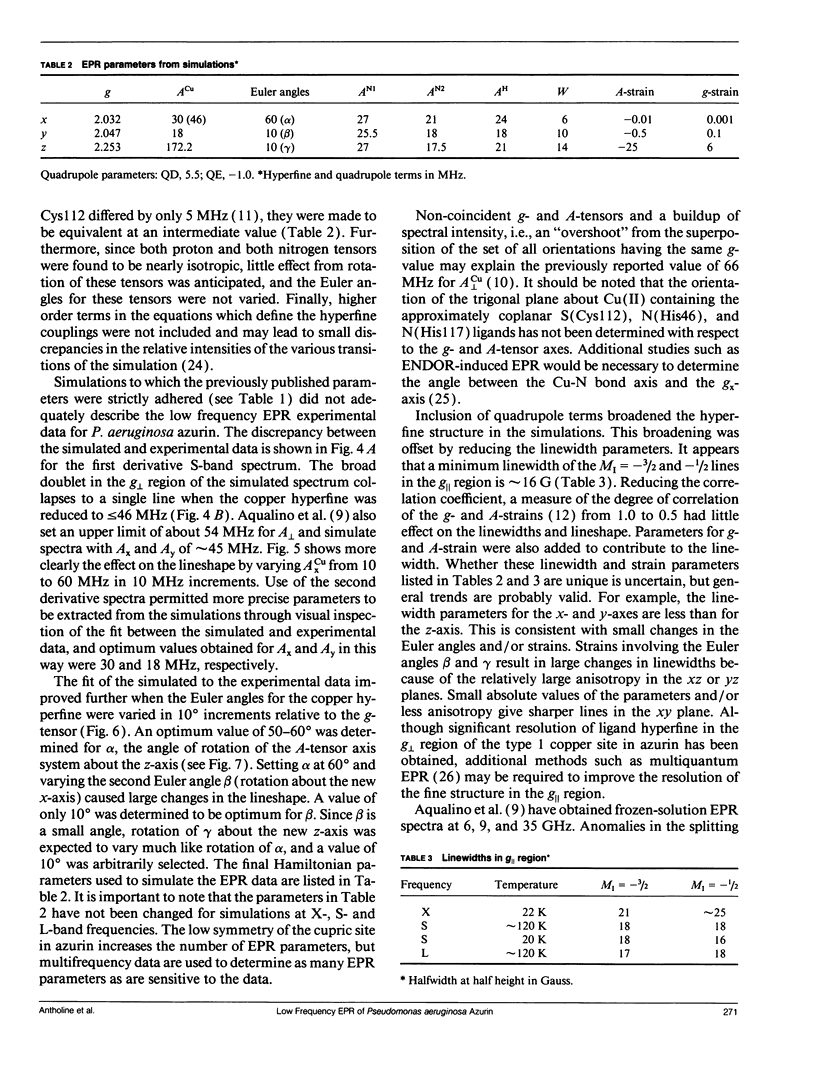
The type 1 copper in Pseudomonas aeruginosa azurin was studied by electron paramagnetic resonance (EPR) spectroscopy at low microwave frequencies. Partially resolved ligand hyperfine structure was observed in the perpendicular region of the spectra at both S-band (2.4 GHz) and L-band (1.1 GHz). A trial and error method, requiring several hundred simulations, has been used to simulate the low frequency EPR data and yield an optimum value of 30 MHz for ACUx, more than one half that previously reported. The fit between the simulated and experimental data is sensitive to changes in the Euler angles and, in particular, to the angle alpha which rotates the Cu A-tensor about the z-axis. Thus, the A- and g-tensors for copper in P. aeruginosa azurin do not appear to be coincident. A value for the Euler angle beta of at least 10 degrees does not disturb the fit between the simulated and experimental data. These studies demonstrate the advantage of evaluating EPR parameters from simulations at more than one frequency, especially at low frequencies where ligand superhyperfine structure may be resolved for type 1 copper.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T. Copper protein structures. Adv Protein Chem. 1991;42:145–197. doi: 10.1016/s0065-3233(08)60536-7. [DOI] [PubMed] [Google Scholar]
- Adman E. T., Stenkamp R. E., Sieker L. C., Jensen L. H. A crystallographic model for azurin a 3 A resolution. J Mol Biol. 1978 Jul 25;123(1):35–47. doi: 10.1016/0022-2836(78)90375-3. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqualino A, Brill AS, Bryce GF, Gerstman BS. Correlated distributions in g and A tensors at a biologically active low-symmetry cupric site. Phys Rev A. 1991 Oct 15;44(8):5257–5271. doi: 10.1103/physreva.44.5257. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of azurin from Alcaligenes denitrificans refinement at 1.8 A resolution and comparison of the two crystallographically independent molecules. J Mol Biol. 1988 Oct 20;203(4):1071–1095. doi: 10.1016/0022-2836(88)90129-5. [DOI] [PubMed] [Google Scholar]
- Blaszak J. A., McMillin D. R., Thornton A. T., Tennent D. L. Kinetics of copper(II) uptake by apoazurin in complexing media. J Biol Chem. 1983 Aug 25;258(16):9886–9892. [PubMed] [Google Scholar]
- Groeneveld C. M., Aasa R., Reinhammar B., Canters G. W. EPR of azurins from Pseudomonas aeruginosa and Alcaligenes denitrificans demonstrates pH-dependence of the copper-site geometry in Pseudomonas aeruginosa protein. J Inorg Biochem. 1987 Oct;31(2):143–154. doi: 10.1016/0162-0134(87)80059-4. [DOI] [PubMed] [Google Scholar]
- Hanna P. M., McMillin D. R., Pasenkiewicz-Gierula M., Antholine W. E., Reinhammar B. Type 2-depleted fungal laccase. Biochem J. 1988 Jul 15;253(2):561–568. doi: 10.1042/bj2530561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnik C. M., Szabo A. G. Confirmation that multiexponential fluorescence decay behavior of holoazurin originates from conformational heterogeneity. Biochemistry. 1989 May 2;28(9):3923–3934. doi: 10.1021/bi00435a045. [DOI] [PubMed] [Google Scholar]
- McMillin D. R., Rosenberg R. C., Gray H. B. Preparation and spectroscopic studies of cobalt(II) derivatives of blue copper proteins. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4760–4762. doi: 10.1073/pnas.71.12.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nar H., Messerschmidt A., Huber R., van de Kamp M., Canters G. W. X-ray crystal structure of the two site-specific mutants His35Gln and His35Leu of azurin from Pseudomonas aeruginosa. J Mol Biol. 1991 Mar 20;218(2):427–447. doi: 10.1016/0022-2836(91)90723-j. [DOI] [PubMed] [Google Scholar]


