Abstract
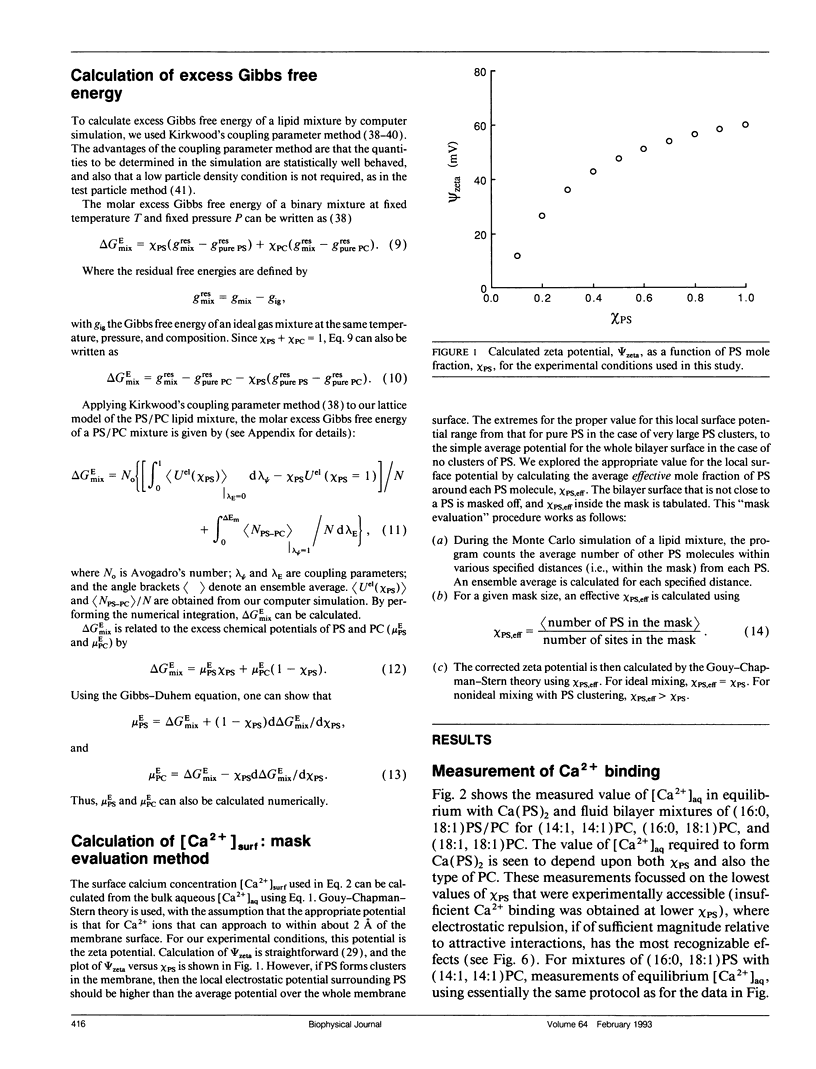
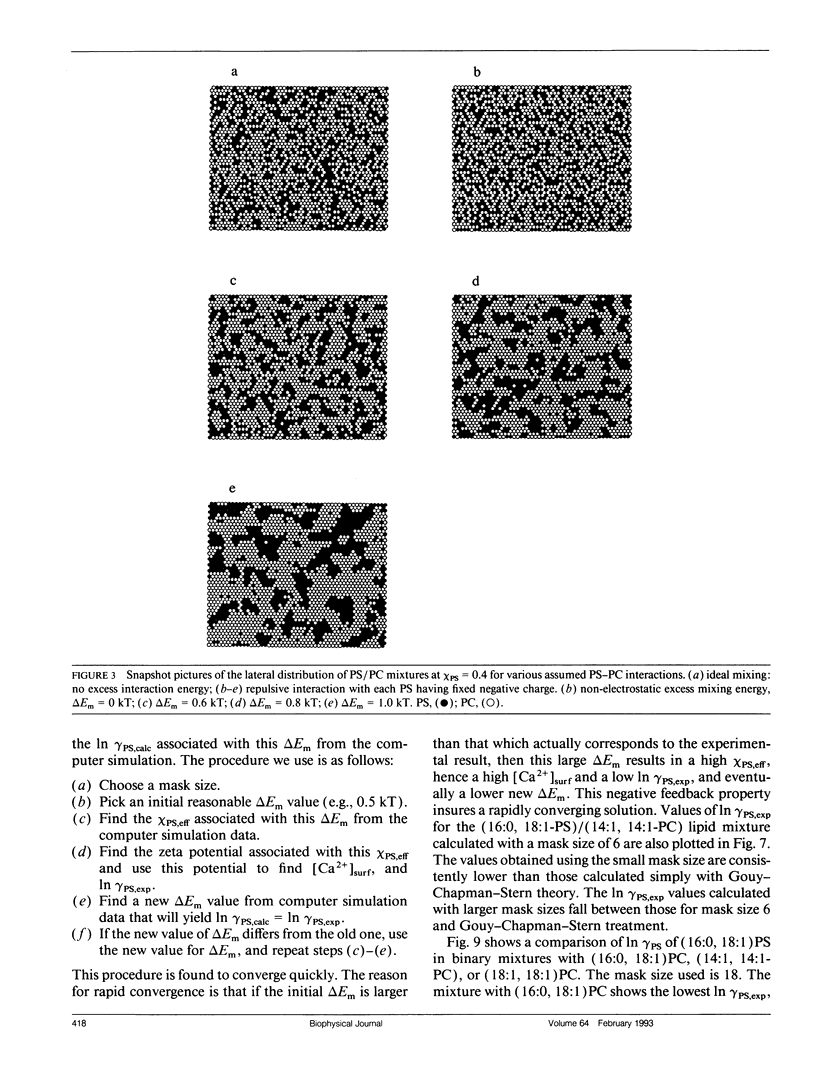
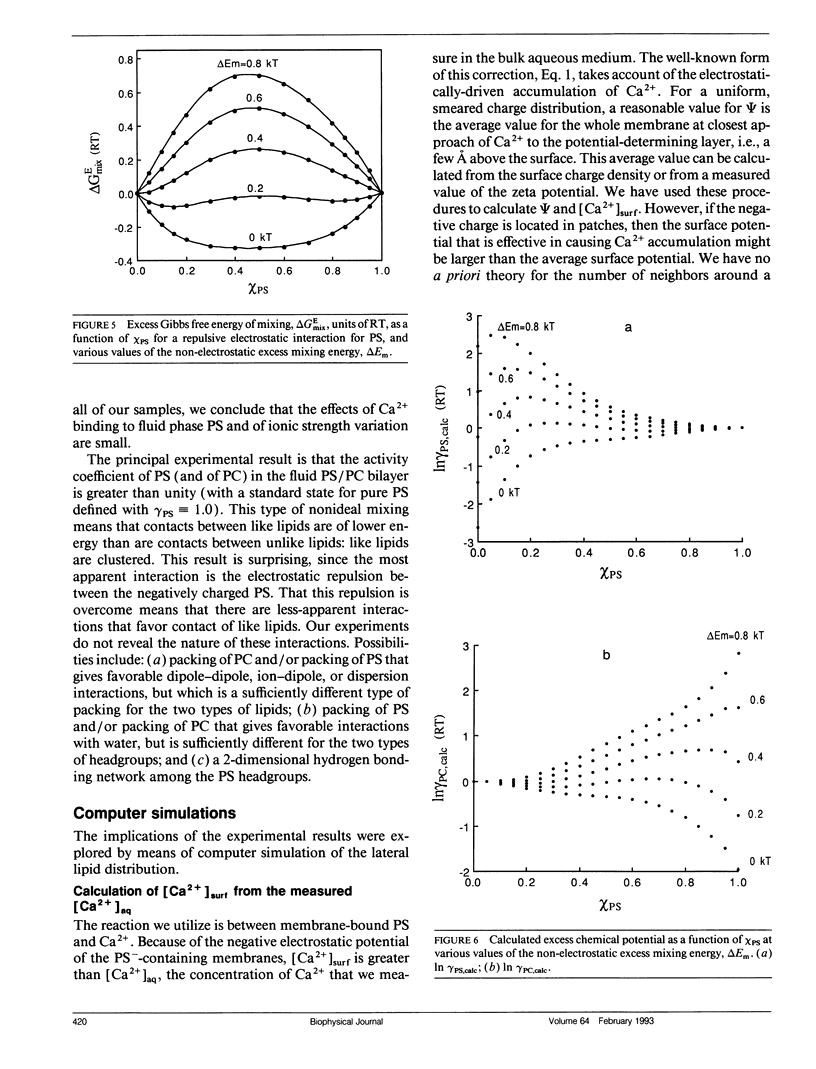
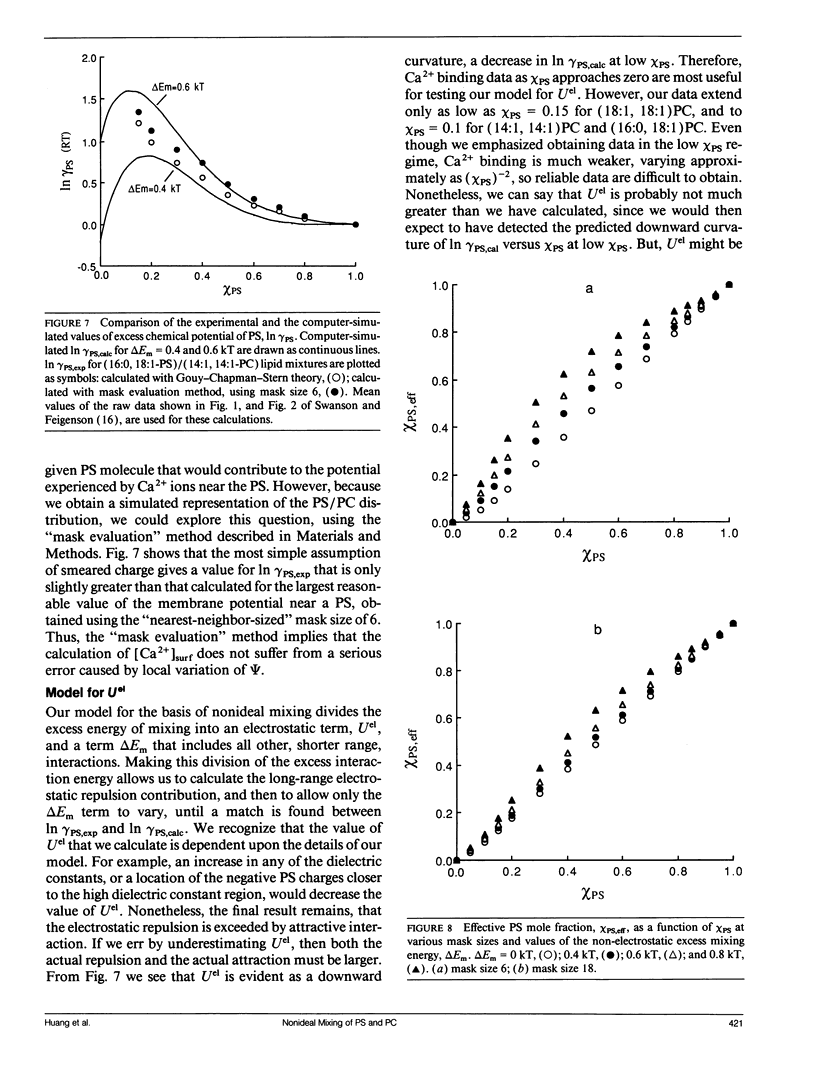
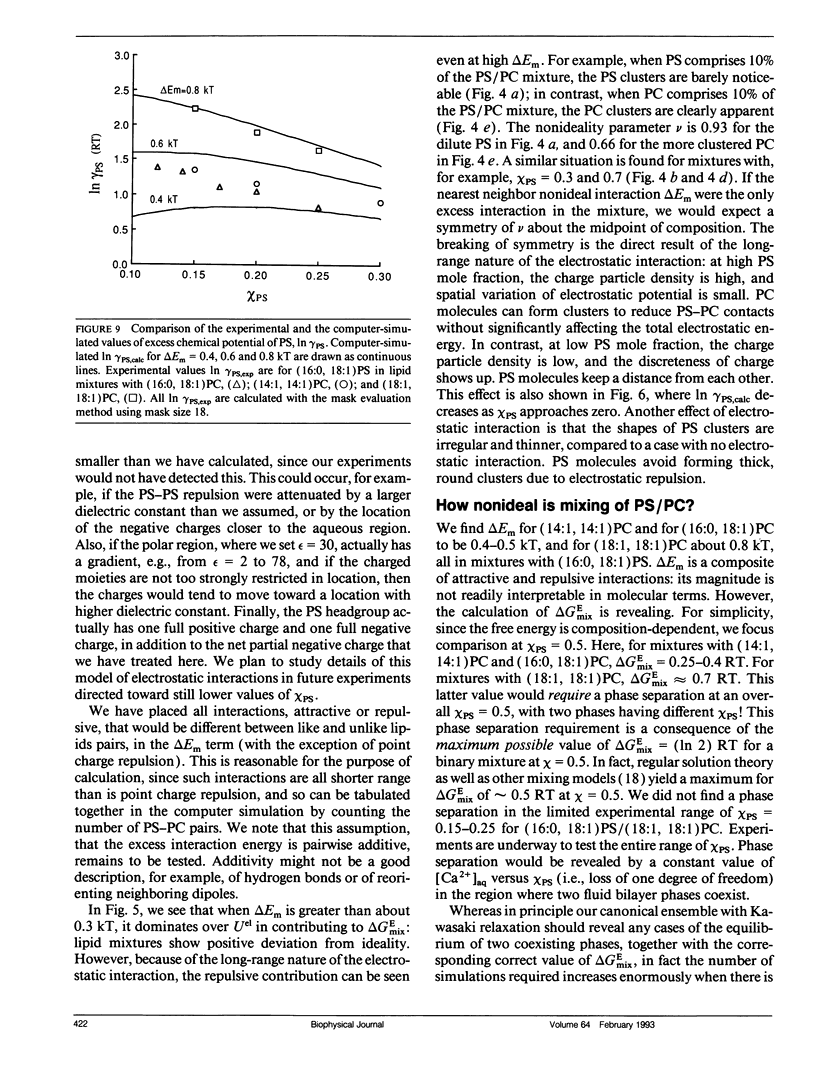
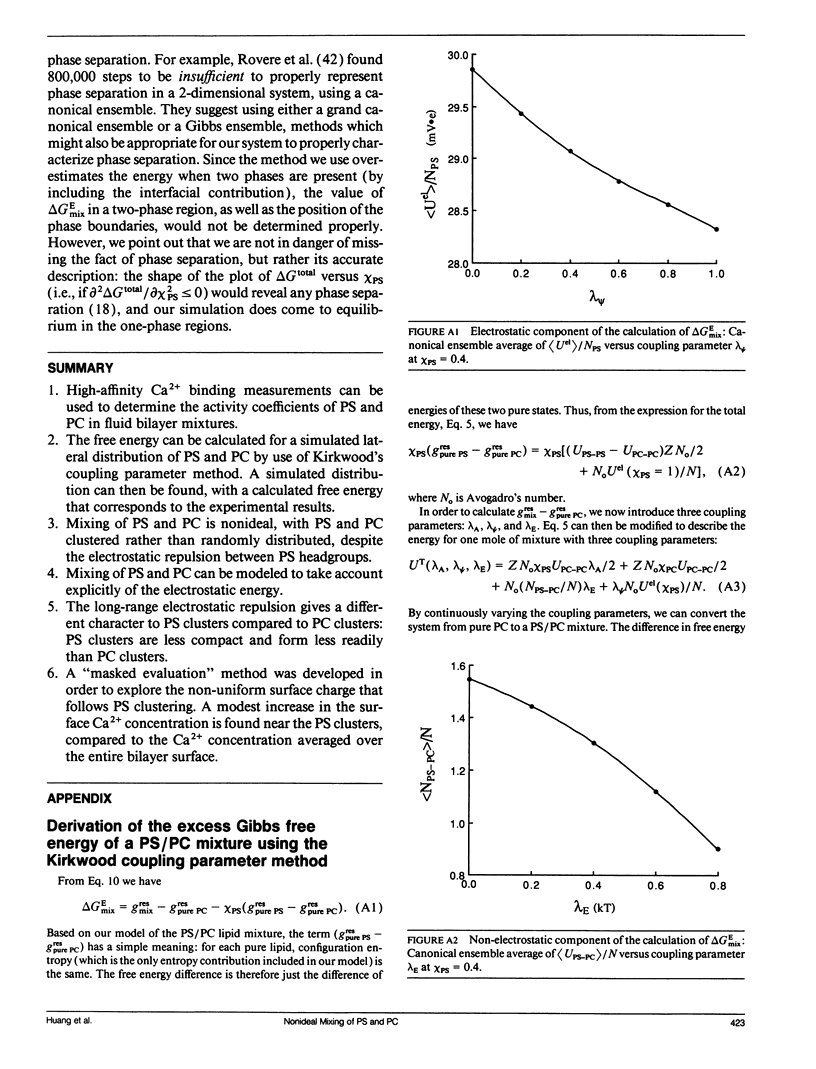
The mixing of phosphatidylserine (PS) and phosphatidylcholine (PC) in fluid bilayer model membranes was studied by measuring binding of aqueous Ca2+ ions. The measured [Ca2+]aq was used to derive the activity coefficient for PS, gamma PS, in the lipid mixture. For (16:0, 18:1) PS in binary mixtures with either (16:0, 18:1)PC, (14:1, 14:1)PC, or (18:1, 18:1)PC, gamma PS > 1; i.e., mixing is nonideal, with PS and PC clustered rather than randomly distributed, despite the electrostatic repulsion between PS headgroups. To understand better this mixing behavior, Monte Carlo simulations of the PS/PC distributions were performed, using Kawasaki relaxation. The excess energy was divided into an electrostatic term Uel and one adjustable term including all other nonideal energy contributions, delta Em. Uel was calculated using a discrete charge theory. Kirkwood's coupling parameter method was used to calculate the excess free energy of mixing, delta GEmix, hence In gamma PS,calc. The values of In gamma PS,calc were equalized by adjusting delta Em in order to find the simulated PS/PC distribution that corresponded to the experimental results. We were thus able to compare the smeared charge calculation of [Ca2+]surf with a calculation ("masked evaluation method") that recognized clustering of the negatively charged PS: clustering was found to have a modest effect on [Ca2+]surf, relative to the smeared charge model. Even though both PS and PC tend to cluster, the long-range nature of the electrostatic repulsion reduces the extent of PS clustering at low PS mole fraction compared to PC clustering at an equivalent low PC mole fraction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Jr Membrane surface charge: discrete and uniform modelling. Prog Biophys Mol Biol. 1974;28:341–370. doi: 10.1016/0079-6107(74)90021-2. [DOI] [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Cevc G., Watts A., Marsh D. Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head-group hydration. Biochemistry. 1981 Aug 18;20(17):4955–4965. doi: 10.1021/bi00520a023. [DOI] [PubMed] [Google Scholar]
- Cheng W. H. A theoretical description of phase diagrams for nonideal lipid mixtures. Biochim Biophys Acta. 1980 Aug 4;600(2):358–366. doi: 10.1016/0005-2736(80)90439-3. [DOI] [PubMed] [Google Scholar]
- Duniec J. T., Thorne S. W. Electrostatic potentials in membrane systems. Bull Math Biol. 1983;45(1):69–90. doi: 10.1007/BF02459388. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Fromherz P. Lipid coumarin dye as a probe of interfacial electrical potential in biomembranes. Methods Enzymol. 1989;171:376–387. doi: 10.1016/s0076-6879(89)71021-1. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G. Modelling the phase equilibria in two-component membranes of phospholipids with different acyl-chain lengths. Biochim Biophys Acta. 1988 Oct 6;944(2):121–134. doi: 10.1016/0005-2736(88)90425-7. [DOI] [PubMed] [Google Scholar]
- Jan N., Lookman T., Pink D. A. On computer simulation methods used to study models of two-component lipid bilayers. Biochemistry. 1984 Jul 3;23(14):3227–3231. doi: 10.1021/bi00309a017. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991 Mar;57(2-3):375–399. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. II. Mictures involving lipids. Biochim Biophys Acta. 1977 Nov 14;472(3-4):285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin A. C. Phosphorus-31 and carbon-13 nuclear magnetic resonance studies of divalent cation binding to phosphatidylserine membranes: use of cobalt as a paramagnetic probe. Biochemistry. 1982 Sep 28;21(20):4879–4885. doi: 10.1021/bi00263a008. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wiener M. C. Structure of fully hydrated bilayer dispersions. Biochim Biophys Acta. 1988 Jul 7;942(1):1–10. doi: 10.1016/0005-2736(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Nelson A. P., McQuarrie D. A. The effect of discrete charges on the electrical properties of a membrane. I. J Theor Biol. 1975 Nov;55(1):13–27. doi: 10.1016/s0022-5193(75)80106-8. [DOI] [PubMed] [Google Scholar]
- Sauvé R., Ohki S. Interactions of divalent cations with negatively charged membrane surfaces. I. Discrete charge potential. J Theor Biol. 1979 Nov 21;81(2):157–179. doi: 10.1016/0022-5193(79)90158-9. [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Freed J. H. Dynamic imaging of lateral diffusion by electron spin resonance and study of rotational dynamics in model membranes. Effect of cholesterol. Biophys J. 1989 Mar;55(3):537–550. doi: 10.1016/S0006-3495(89)82847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. E., Feigenson G. W. Thermodynamics of mixing of phosphatidylserine/phosphatidylcholine from measurements of high-affinity calcium binding. Biochemistry. 1990 Sep 11;29(36):8291–8297. doi: 10.1021/bi00488a013. [DOI] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]


