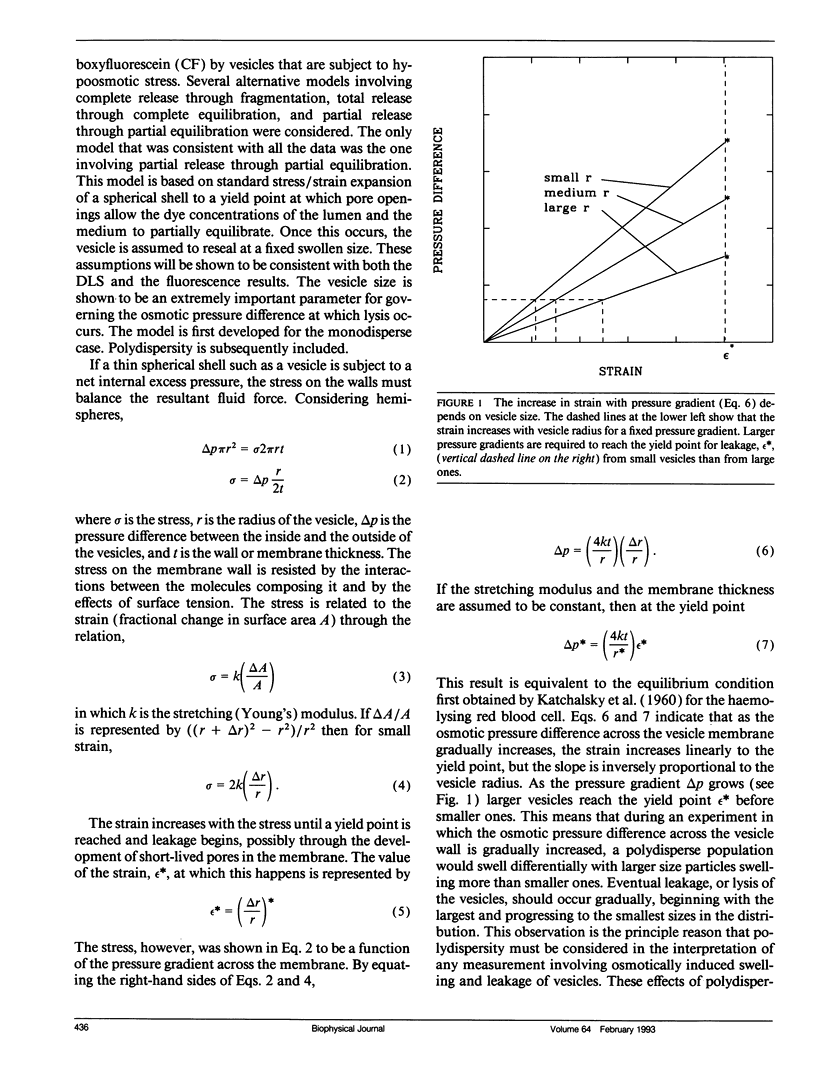
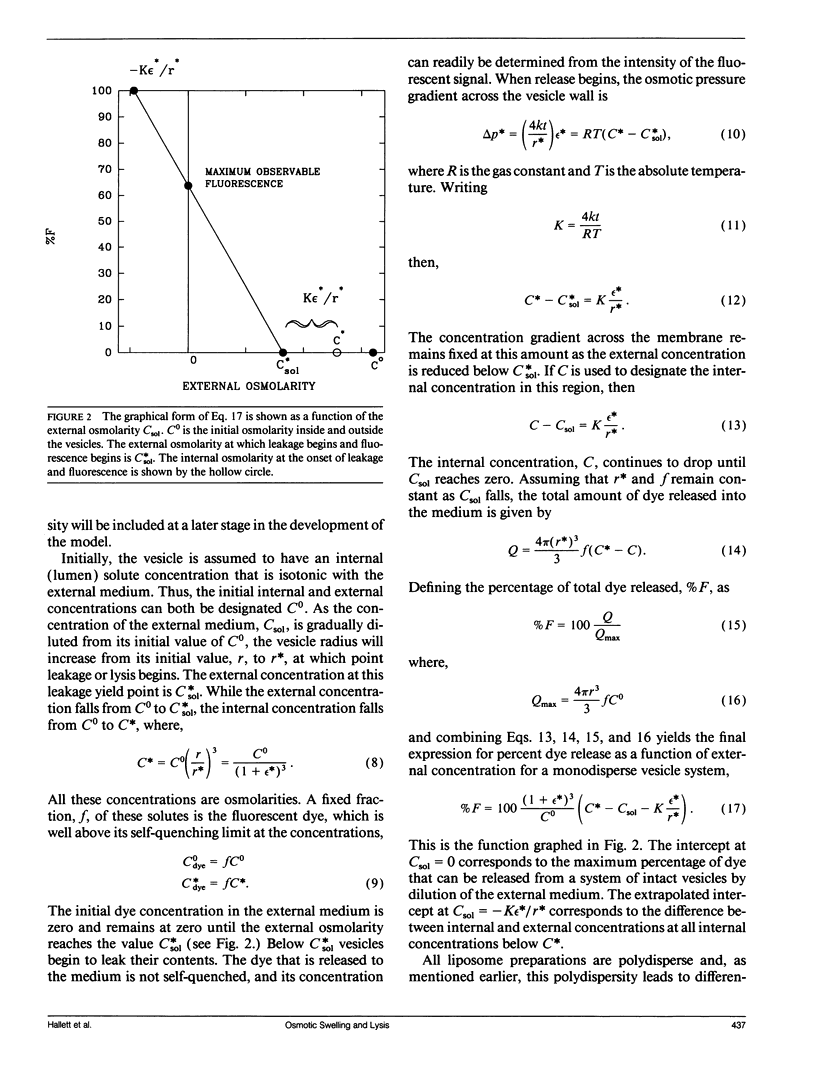
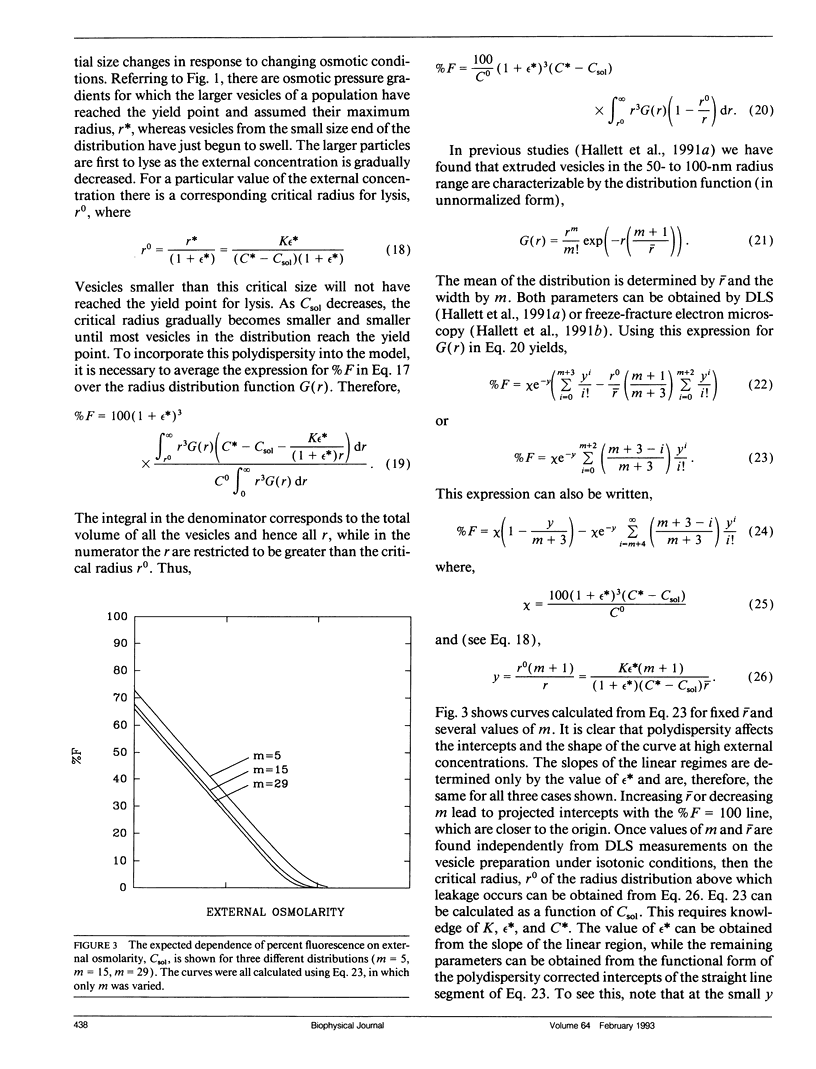
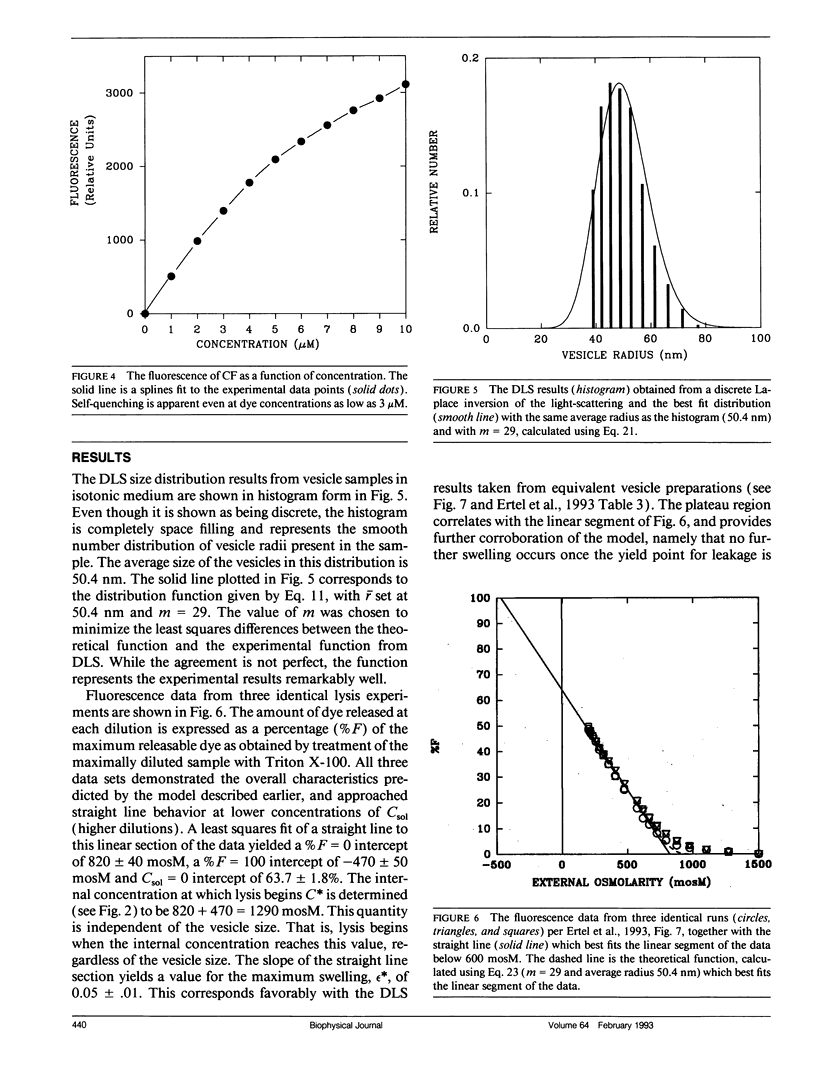
Abstract
Vesicle polydispersity and leakage of solutes from the vesicle lumen influence the measurement and analysis of osmotically induced vesicle swelling and lysis, but their effects have not been considered in previous studies of these processes. In this study, a model is developed which expressly includes polydispersity and leakage effects. The companion paper demonstrated the preparation and characterization of large unilamellar lipid vesicles. A dye release technique was employed to indicate the leakage of solutes from the vesicles during osmotic swelling. Changes in vesicle size were monitored by dynamic light scattering (DLS). In explaining the results, the model identifies three stages. The first phase involves differential increases in membrane tension with strain increasing in larger vesicles before smaller ones. In the second phase, the yield point for lysis (leakage) is reached sequentially from large sizes to small sizes. In the final phase, the lumen contents and the external medium partially equilibrate under conditions of constant membrane tension. When fit to the data, the model yields information on polydispersity-corrected values for membrane area compressibility, Young's modulus, and yield point for lysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergelson L. D., Barsukov L. I. Topological asymmetry of phospholipids in membranes. Science. 1977 Jul 15;197(4300):224–230. doi: 10.1126/science.327544. [DOI] [PubMed] [Google Scholar]
- Booth I. R., Cairney J., Sutherland L., Higgin C. F. Enteric bacteria and osmotic stress: an integrated homeostatic system. Soc Appl Bacteriol Symp Ser. 1988;17:35S–49S. [PubMed] [Google Scholar]
- Chen R. F., Knutson J. R. Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes: energy transfer to nonfluorescent dimers. Anal Biochem. 1988 Jul;172(1):61–77. doi: 10.1016/0003-2697(88)90412-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol. 1991 Jan;119(1):1–13. doi: 10.1007/BF01868535. [DOI] [PubMed] [Google Scholar]
- Hallett F. R., Nickel B., Samuels C., Krygsman P. H. Determination of vesicle size distributions by freeze-fracture electron microscopy. J Electron Microsc Tech. 1991 Apr;17(4):459–466. doi: 10.1002/jemt.1060170409. [DOI] [PubMed] [Google Scholar]
- Hallett F. R., Watton J., Krygsman P. Vesicle sizing: Number distributions by dynamic light scattering. Biophys J. 1991 Feb;59(2):357–362. doi: 10.1016/S0006-3495(91)82229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantz E., Cao A., Escaig J., Taillandier E. The osmotic response of large unilamellar vesicles studied by quasielastic light scattering. Biochim Biophys Acta. 1986 Nov 17;862(2):379–386. doi: 10.1016/0005-2736(86)90241-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Active transport in Escherichia coli: passage to permease. Annu Rev Biophys Biophys Chem. 1986;15:279–319. doi: 10.1146/annurev.bb.15.060186.001431. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986 Jun 13;858(1):161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Rutkowski C. A., Williams L. M., Haines T. H., Cummins H. Z. The elasticity of synthetic phospholipid vesicles obtained by photon correlation spectroscopy. Biochemistry. 1991 Jun 11;30(23):5688–5696. doi: 10.1021/bi00237a008. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Ye R. G., Verkman A. S. Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochemistry. 1989 Jan 24;28(2):824–829. doi: 10.1021/bi00428a062. [DOI] [PubMed] [Google Scholar]


