Abstract
The catalytic role of the cofactor phosphate moiety at the active site of glycogen phosphorylase has been the subject of many investigations including solution-state high-resolution 31P-NMR studies. In this study the pyridoxal phosphate moiety in both the inactive and active forms of microcrystalline phosphorylase b has been investigated by high-resolution 31P magic-angle spinning NMR. The symmetry of the shielding tensor in model compounds at varying degrees of ionization is investigated and the results indicate a marked difference between the dianionic and monoanionic model compounds. Consequently the observed similarity in the principal tensor components describing the shielding tensor of the phosphorus nuclei present at the active site of both the R- and T-state conformations suggests that there is no change in ionization site upon activation in contrast to suggestions based upon isotropic shifts. Since previous relaxation measurements have pointed to the need to consider motional influences in such systems, several plausible models are considered. Subject to the assumption of congruency between the principal axis system describing the shielding interaction and molecular frame determined by the molecular symmetry axes, we conclude that the phosphate cofactor is dianionic in both forms.
Full text
PDF

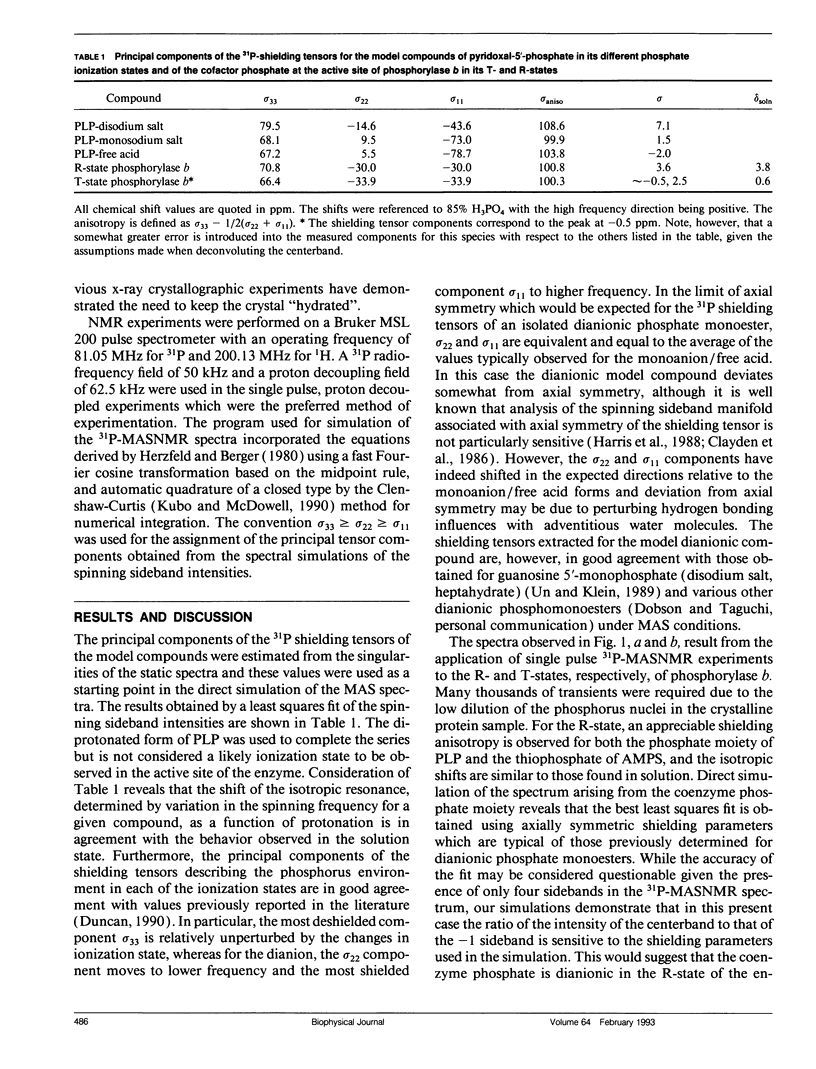




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barford D., Schwabe J. W., Oikonomakos N. G., Acharya K. R., Hajdu J., Papageorgiou A. C., Martin J. L., Knott J. C., Vasella A., Johnson L. N. Channels at the catalytic site of glycogen phosphorylase b: binding and kinetic studies with the beta-glycosidase inhibitor D-gluconohydroximo-1,5-lactone N-phenylurethane. Biochemistry. 1988 Sep 6;27(18):6733–6741. doi: 10.1021/bi00418a014. [DOI] [PubMed] [Google Scholar]
- DiVerdi J. A., Opella S. J. Dynamics of B-DNA in the solid state. J Mol Biol. 1981 Jun 25;149(2):307–311. doi: 10.1016/0022-2836(81)90304-1. [DOI] [PubMed] [Google Scholar]
- Feldmann K., Hull W. E. 31P nuclear magnetic resonance studies of glycogen phosphorylase from rabbit skeletal muscle: ionization states of pyridoxal 5'-phosphate. Proc Natl Acad Sci U S A. 1977 Mar;74(3):856–860. doi: 10.1073/pnas.74.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. G. Letter: Observation of the effect of water on the 31P nuclear magnetic resonance spectra of dipalmitoyllecithin. J Am Chem Soc. 1976 Feb 4;98(3):851–853. doi: 10.1021/ja00419a044. [DOI] [PubMed] [Google Scholar]
- Griffin R. G., Powers L., Pershan P. S. Head-group conformation in phospholipids: a phosphorus-31 nuclear magnetic resonance study of oriented monodomain dipalmitoylphosphatidylcholine bilayers. Biochemistry. 1978 Jul 11;17(14):2718–2722. doi: 10.1021/bi00607a004. [DOI] [PubMed] [Google Scholar]
- Herzfeld J., Griffin R. G., Haberkorn R. A. Phosphorus-31 chemical-shift tensors in barium diethyl phosphate and urea-phosphoric acid: model compounds for phospholipid head-group studies. Biochemistry. 1978 Jul 11;17(14):2711–2718. doi: 10.1021/bi00607a003. [DOI] [PubMed] [Google Scholar]
- Hoerl M., Feldmann K., Schnackerz K. D., Helmreich E. J. Ionization of pyridoxal 5'-phosphate and the interactions of AMP-S and thiophosphoseryl residues in native and succinylated rabbit muscle glycogen phosphorylase b and a as inferred from 31P NMR spectra. Biochemistry. 1979 Jun 12;18(12):2457–2464. doi: 10.1021/bi00579a004. [DOI] [PubMed] [Google Scholar]
- Johnson L. N., Barford D. Glycogen phosphorylase. The structural basis of the allosteric response and comparison with other allosteric proteins. J Biol Chem. 1990 Feb 15;265(5):2409–2412. [PubMed] [Google Scholar]
- Johnson L. N., Madsen N. B., Mosley J., Wilson K. S. The crystal structure of phosphorylase beta at 6 A resolution. J Mol Biol. 1974 Dec 25;90(4):703–717. doi: 10.1016/0022-2836(74)90534-8. [DOI] [PubMed] [Google Scholar]
- Kasvinsky P. J., Madsen N. B. Activity of glycogen phosphorylase in the crystalline state. J Biol Chem. 1976 Nov 10;251(21):6852–6859. [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. 31P nuclear magnetic resonance chemical shielding tensors of phosphorylethanolamine, lecithin, and related compounds: Applications to head-group motion in model membranes. Biochemistry. 1976 Mar 9;15(5):967–974. doi: 10.1021/bi00650a004. [DOI] [PubMed] [Google Scholar]
- McDermott A. E., Creuzet F., Griffin R. G., Zawadzke L. E., Ye Q. Z., Walsh C. T. Rotational resonance determination of the structure of an enzyme-inhibitor complex: phosphorylation of an (aminoalkyl)phosphinate inhibitor of D-alanyl-D-alanine ligase by ATP. Biochemistry. 1990 Jun 19;29(24):5767–5775. doi: 10.1021/bi00476a018. [DOI] [PubMed] [Google Scholar]
- Opella S. J., Wise W. B., DiVerdi J. A. Deoxyribonucleic acid dynamics from phosphorus-31 nuclear magnetic resonance. Biochemistry. 1981 Jan 20;20(2):284–290. doi: 10.1021/bi00505a009. [DOI] [PubMed] [Google Scholar]
- Opella S. J., Wise W. B., DiVerdi J. A. Deoxyribonucleic acid dynamics from phosphorus-31 nuclear magnetic resonance. Biochemistry. 1981 Jan 20;20(2):284–290. doi: 10.1021/bi00505a009. [DOI] [PubMed] [Google Scholar]
- Shindo H., Zimmerman S. B. Sequence-dependent variations in the backbone geometry of a synthetic DNA fibre. Nature. 1980 Feb 14;283(5748):690–691. doi: 10.1038/283690a0. [DOI] [PubMed] [Google Scholar]
- Sprang S., Fletterick R. J. The structure of glycogen phosphorylase alpha at 2.5 A resolution. J Mol Biol. 1979 Jul 5;131(3):523–551. doi: 10.1016/0022-2836(79)90006-8. [DOI] [PubMed] [Google Scholar]
- Terao T., Matsui S., Akasaka K. 31P chemical shift anisotropy in solid nucleic acids. J Am Chem Soc. 1977 Aug 31;99(18):6136–6138. doi: 10.1021/ja00460a067. [DOI] [PubMed] [Google Scholar]
- Viswamitra M. A., Reddy B. S., Lin G. H., Sundaralingam M. Stereochemistry of nucleic acids and their constituents. XVII. Crystal and molecular structure of deoxycytidine 5'-phosphate monohydrate. A possible puckering for the furanoside ring in B-deoxyribonucleic acid. J Am Chem Soc. 1971 Sep 8;93(18):4565–4573. doi: 10.1021/ja00747a038. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sprang S. R., Fletterick R. J. Catalytic site of glycogen phosphorylase: structural changes during activation and mechanistic implications. Biochemistry. 1982 Oct 12;21(21):5372–5382. doi: 10.1021/bi00264a039. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. 31P NMR relaxation studies of the activation of the coenzyme phosphate of glycogen phosphorylase. The role of motion of the bound phosphate. Biophys J. 1985 Dec;48(6):1019–1026. doi: 10.1016/S0006-3495(85)83864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. Active form of pyridoxal phosphate in glycogen phosphorylase. Phosphorus-31 nuclear magentic resonance investigation. Biochemistry. 1981 Mar 31;20(7):1748–1756. doi: 10.1021/bi00510a007. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. Covalently activated glycogen phosphorylase: a phosphorus-31 nuclear magnetic resonance and ultracentrifugation analysis. Biochemistry. 1982 Dec 21;21(26):6716–6722. doi: 10.1021/bi00269a016. [DOI] [PubMed] [Google Scholar]


