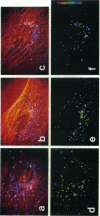
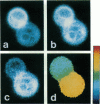
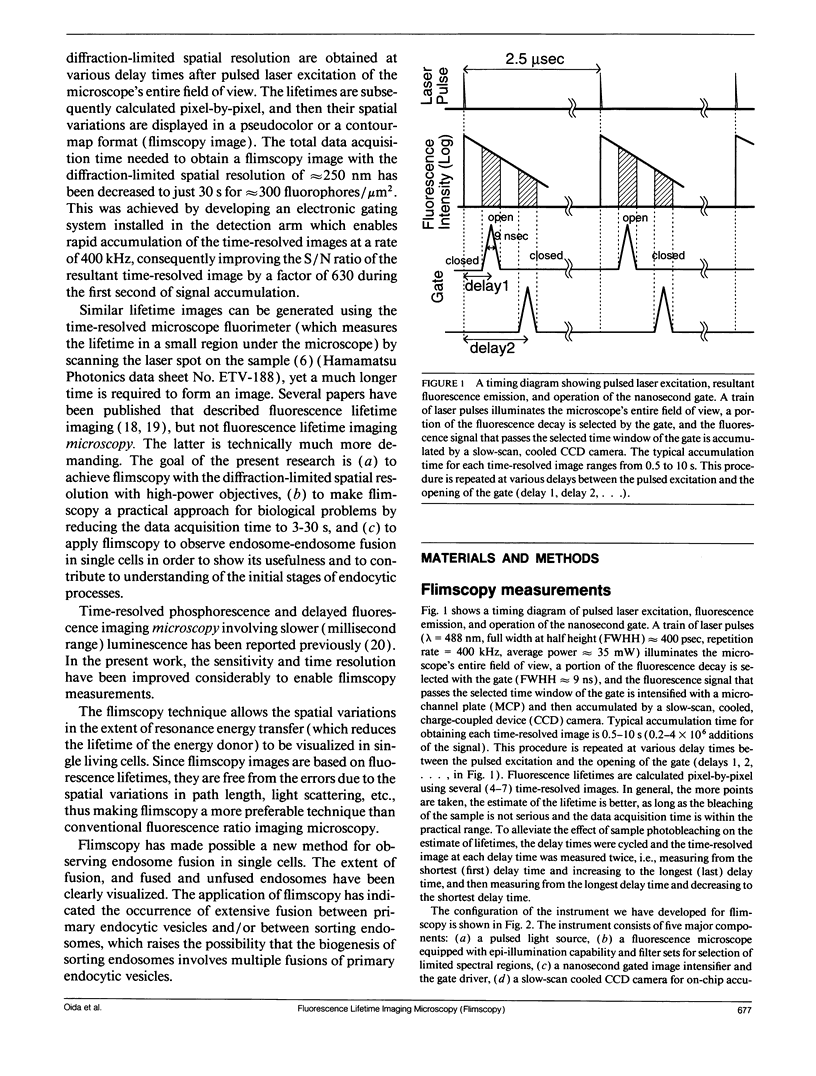
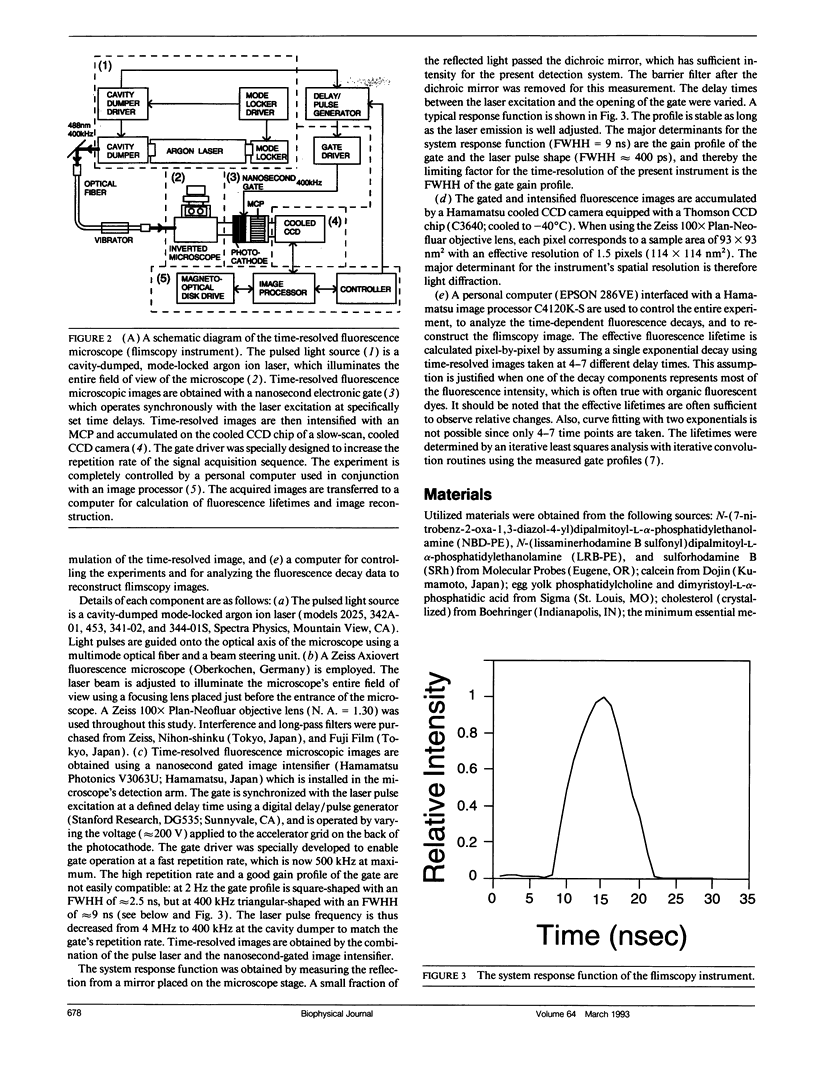
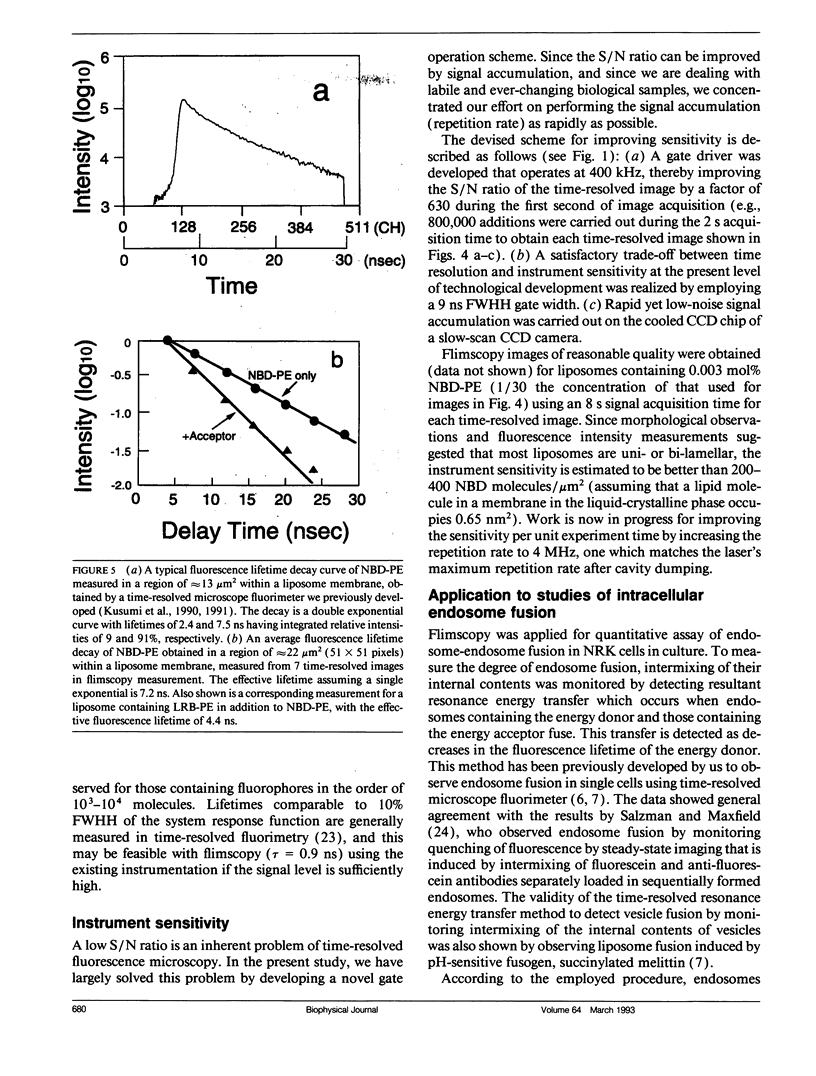
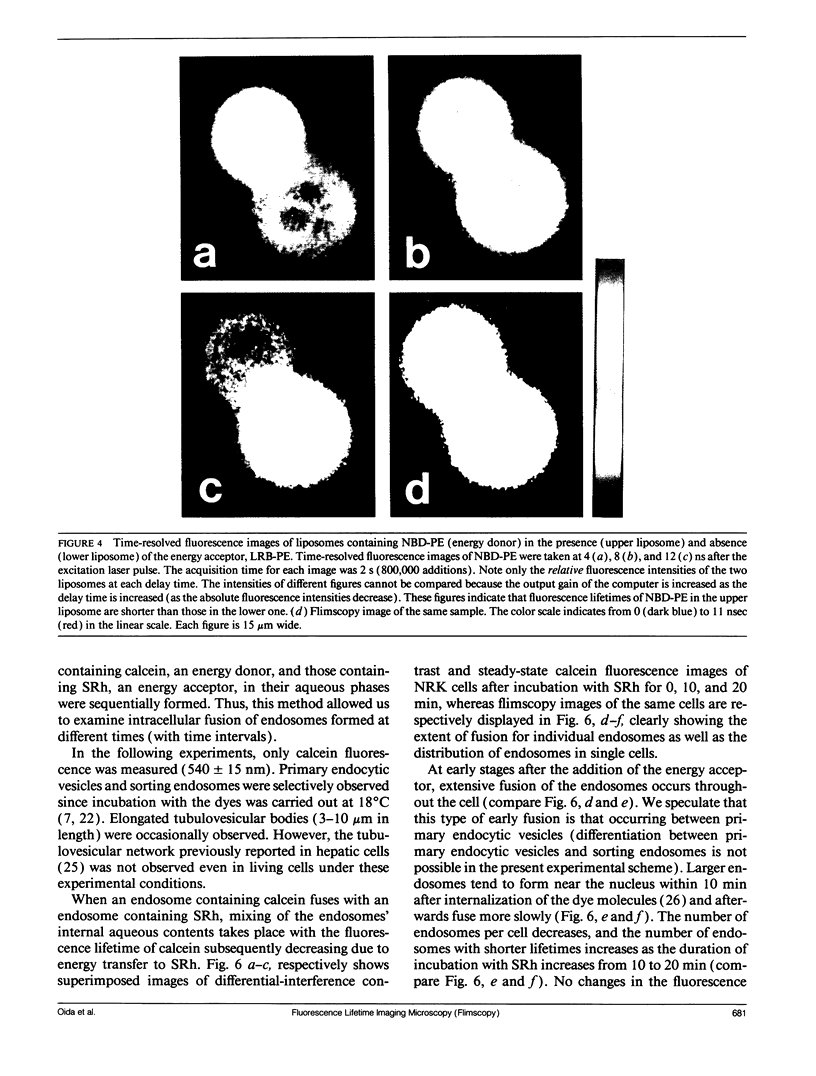
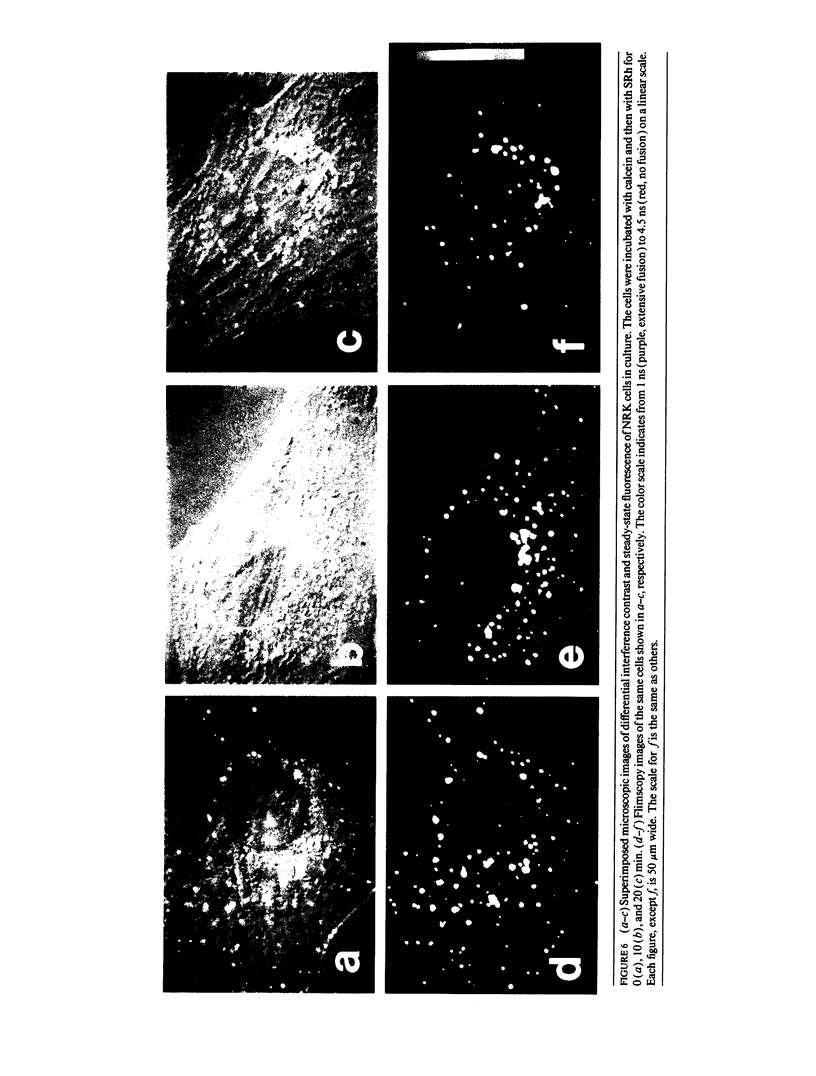
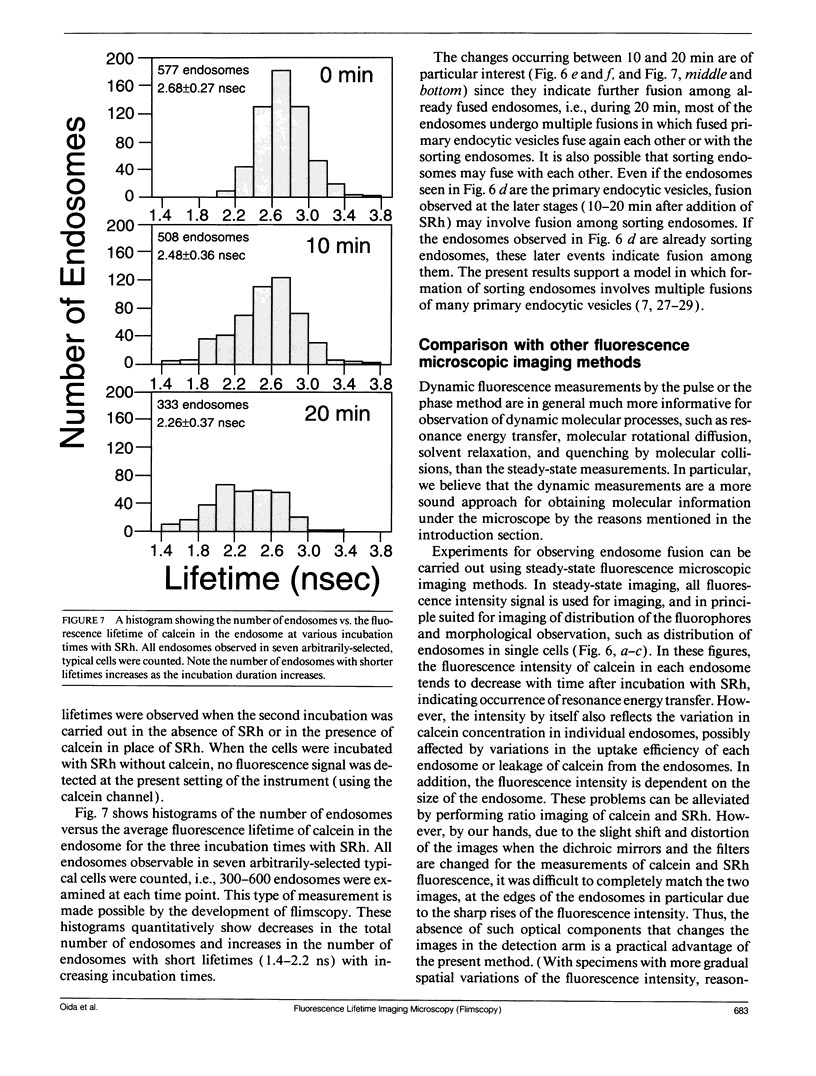
Abstract
A new method of fluorescence microscopy for cell imaging has been developed that takes advantage of the spatial variations of fluorescence lifetimes in single cells as a source of image contrast, and thus it is named "fluorescence lifetime imaging microscopy (flimscopy)". Since time-resolved fluorescence measurements are sensitive to molecular dynamics and interactions, flimscopy allows the molecular information to be visualized in single cells. In flimscopy measurements, several (nanosecond) time-resolved fluorescence images of a sample are obtained at various delay times after pulsed laser excitation of the microscope's entire field of view. Lifetimes are calculated pixel-by-pixel from these time-resolved images, and the spatial variations of the lifetimes are then displayed in a pseudocolor format (flimscopy image). The total data acquisition time needed to obtain a flimscopy image with the diffraction-limited spatial resolution (approximately 250 nm) is decreased to just approximately 30 s for approximately 300 fluorescent molecules/micron2. This was achieved by developing a high-frequency (400 kHz) nanosecond-gating (9 ns full width at half height)-signal accumulation system. This technique allows the extent of resonance energy transfer to be visualized in single living cells, and is free from the errors due to variations in path length, light scattering, and the number of fluorophores that necessitate complex corrections in steady-state microfluorometry and fluorescence ratio imaging microscopy. Flimscopy was applied here to observe the extent of fusion of individual endosomes in single cells. Results revealed the occurrence of extensive fusion between primary endocytic vesicles and/or sorting endosomes, thereby raising the possibility that the biogenesis of sorting endosomes involves multiple fusions of primary endocytic vesicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Harootunian A. T., Buechler Y. J., Taylor S. S., Tsien R. Y. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991 Feb 21;349(6311):694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- Bottiroli G., Cionini P. G., Docchio F., Sacchi C. A. In situ evaluation of the functional state of chromatin by means of Quinacrine Mustard staining and time-resolved fluorescence microscopy. Histochem J. 1984 Mar;16(3):223–233. doi: 10.1007/BF01003607. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Nuydens R., Geerts H., Hopkins C. R. Dynamic behavior of the transferrin receptor followed in living epidermoid carcinoma (A431) cells with nanovid microscopy. Cell Motil Cytoskeleton. 1988;9(1):30–47. doi: 10.1002/cm.970090105. [DOI] [PubMed] [Google Scholar]
- Docchio F., Ramponi R., Sacchi C. A., Bottiroli G., Freitas I. Time-resolved fluorescence microscopy of hematoporphyrin-derivative in cells. Lasers Surg Med. 1982;2(1):21–28. doi: 10.1002/lsm.1900020103. [DOI] [PubMed] [Google Scholar]
- Dunn K. W., Maxfield F. R. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992 Apr;117(2):301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Fernandez S. M., Berlin R. D. Cell surface distribution of lectin receptors determined by resonance energy transfer. Nature. 1976 Dec 2;264(5585):411–415. doi: 10.1038/264411a0. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Gruenberg J. The arguments for pre-existing early and late endosomes. Trends Cell Biol. 1991 Jul;1(1):5–9. doi: 10.1016/0962-8924(91)90047-d. [DOI] [PubMed] [Google Scholar]
- Herman B. A., Fernandez S. M. A microfluorimetric study of membrane dynamics during development of dystrophic muscle in vitro. Arch Biochem Biophys. 1979 Sep;196(2):430–435. doi: 10.1016/0003-9861(79)90294-7. [DOI] [PubMed] [Google Scholar]
- Herman B. A., Fernandez S. M. Changes in membrane dynamics associated with myogenic cell fusion. J Cell Physiol. 1978 Mar;94(3):253–263. doi: 10.1002/jcp.1040940303. [DOI] [PubMed] [Google Scholar]
- Herman B. A., Fernandez S. M. Dynamics and topographical distribution of surface glycoproteins during myoblast fusion: a resonance energy transfer study. Biochemistry. 1982 Jul 6;21(14):3275–3283. doi: 10.1021/bi00257a005. [DOI] [PubMed] [Google Scholar]
- Herman B. Resonance energy transfer microscopy. Methods Cell Biol. 1989;30:219–243. doi: 10.1016/s0091-679x(08)60981-4. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990 Jul 26;346(6282):335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Fluorescent triplet probes for measuring the rotational diffusion of membrane proteins. Biochem J. 1982 Apr 1;203(1):313–321. doi: 10.1042/bj2030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A., Tsuji A., Murata M., Sako Y., Yoshizawa A. C., Kagiwada S., Hayakawa T., Ohnishi S. Development of a streak-camera-based time-resolved microscope fluorimeter and its application to studies of membrane fusion in single cells. Biochemistry. 1991 Jul 2;30(26):6517–6527. doi: 10.1021/bi00240a024. [DOI] [PubMed] [Google Scholar]
- Marriott G., Clegg R. M., Arndt-Jovin D. J., Jovin T. M. Time resolved imaging microscopy. Phosphorescence and delayed fluorescence imaging. Biophys J. 1991 Dec;60(6):1374–1387. doi: 10.1016/S0006-3495(91)82175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. F. Maturation models for endosome and lysosome biogenesis. Trends Cell Biol. 1991 Oct;1(4):77–82. doi: 10.1016/0962-8924(91)90022-2. [DOI] [PubMed] [Google Scholar]
- Sacchi C. A., Svelto O., Prenna G. Pulsed tunable lasers in cytofluorometry. Histochem J. 1974 May;6(3):251–258. doi: 10.1007/BF01312245. [DOI] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Intracellular fusion of sequentially formed endocytic compartments. J Cell Biol. 1988 Apr;106(4):1083–1091. doi: 10.1083/jcb.106.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Uster P. S., Pagano R. E. Resonance energy transfer microscopy: observations of membrane-bound fluorescent probes in model membranes and in living cells. J Cell Biol. 1986 Oct;103(4):1221–1234. doi: 10.1083/jcb.103.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Gaub H. E., McConnell H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy transfer in an evanescent wave-field. Nature. 1986 Mar 13;320(6058):179–181. doi: 10.1038/320179a0. [DOI] [PubMed] [Google Scholar]