Abstract
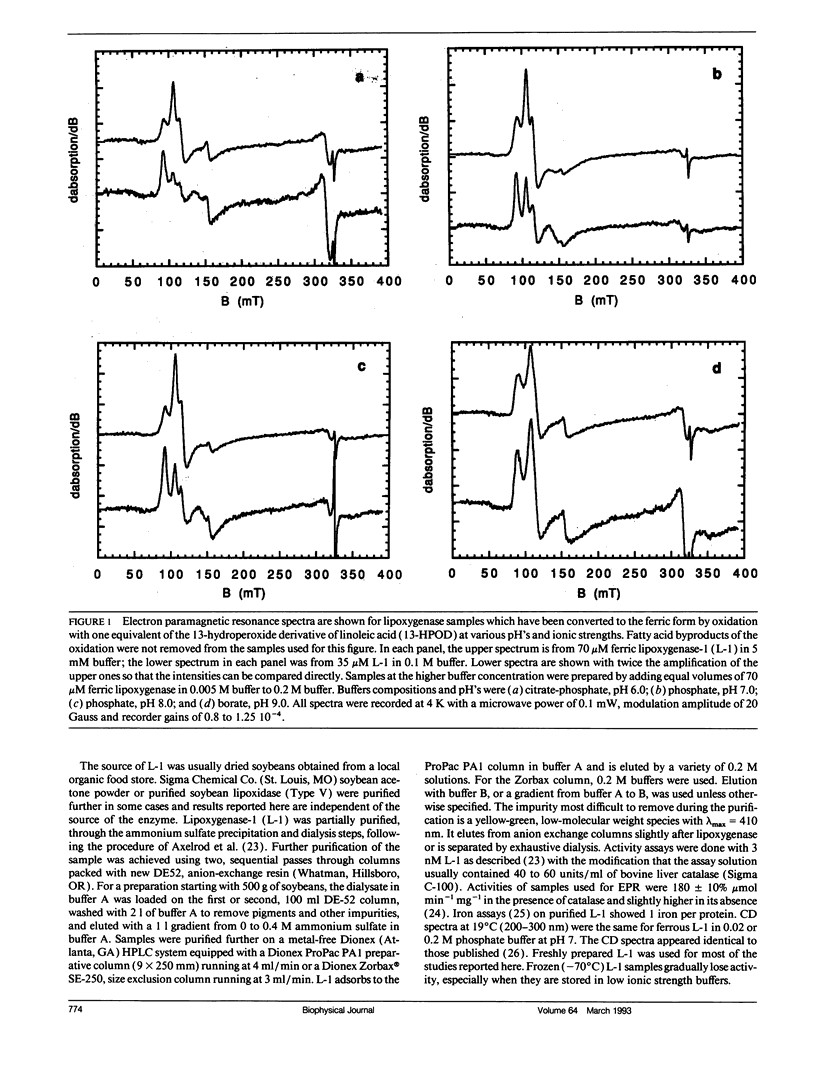
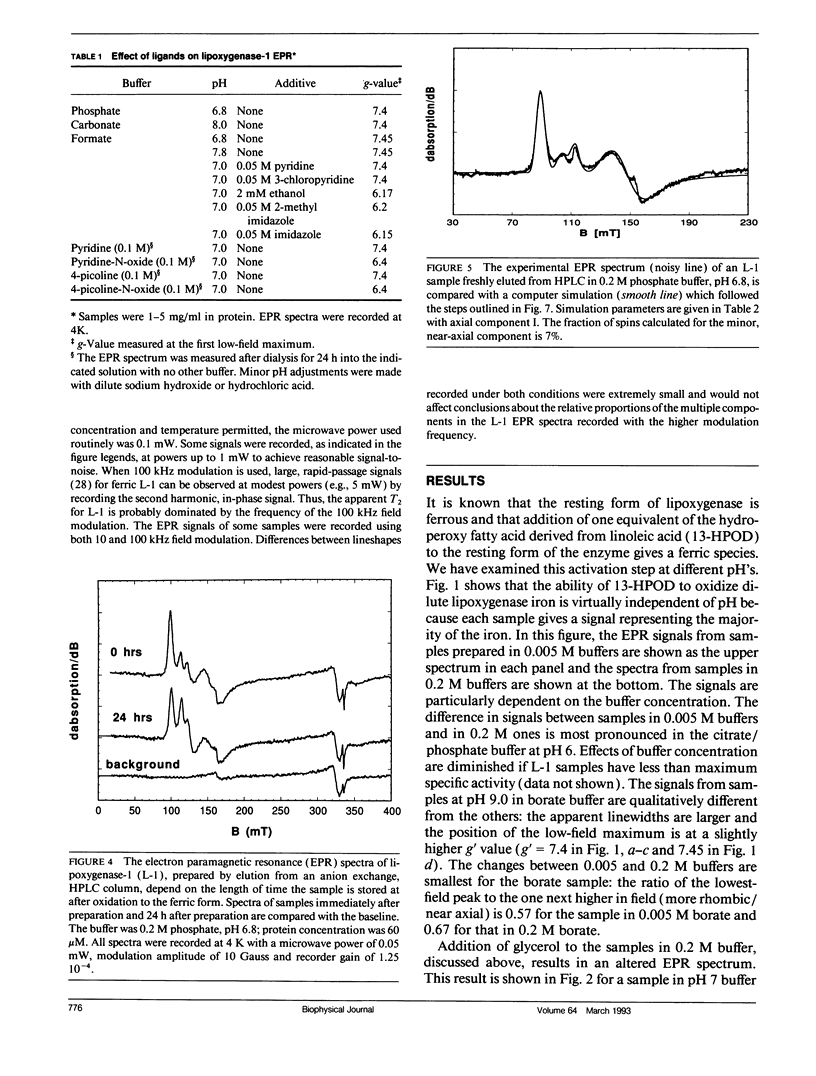
A form of ferric lipoxygenase-1 has been isolated that gives an EPR spectrum that is dominated by a species of intermediate rhombicity (E/D = 0.065). This species is obtained in the presence of a number of buffers of high concentration and in the absence of fatty acid byproducts of the iron oxidation. The species is unstable over a period of one day with respect to symmetry of the iron. The EPR lineshapes of the unstable species are highly sensitive to the anionic composition of the buffer and to the addition of neutral ligands. These results suggest that newly formed ferric lipoxygenase has weak affinity for a number of ligands. Affinity of charged ligands for the iron center may provide a mechanism for charge compensation as the iron center alternates between ferric and ferrous in the catalytic cycle. We use spectral simulation to evaluate quantitatively the interaction of the ferric center with ligands and also show that a transition in the middle Kramers doublet makes a significant contribution to the EPR spectrum of the more rhombic species.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom L. M., Benkovic S. J., Gaffney B. J. Characterization of phenylalanine hydroxylase. Biochemistry. 1986 Jul 29;25(15):4204–4210. doi: 10.1021/bi00363a006. [DOI] [PubMed] [Google Scholar]
- Blum H., Chance B., Litchfield W. J. Effect of pH on bovine liver catalase as determined by electron paramagnetic resonance. Biochim Biophys Acta. 1978 Jun 21;534(2):317–321. doi: 10.1016/0005-2795(78)90014-4. [DOI] [PubMed] [Google Scholar]
- Boyington J. C., Gaffney B. J., Amzel L. M. Crystallization and preliminary x-ray analysis of soybean lipoxygenase-1, a non-heme iron-containing dioxygenase. J Biol Chem. 1990 Aug 5;265(22):12771–12773. [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M., Schewe T., Rapoport S. M. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxy-eicosatetraenoic acid. J Biol Chem. 1982 Jun 10;257(11):6050–6055. [PubMed] [Google Scholar]
- Christopher J., Pistorius E., Axelrod B. Isolation of an isozyme of soybean lipoxygenase. Biochim Biophys Acta. 1970 Jan 14;198(1):12–19. doi: 10.1016/0005-2744(70)90028-8. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Lewis D. F. Activation of 15-lipoxygenase by low density lipoprotein in vascular endothelial cells. Relationship to the oxidative modification of low density lipoprotein. Prostaglandins Leukot Essent Fatty Acids. 1992 Jan;45(1):49–57. doi: 10.1016/0952-3278(92)90102-o. [DOI] [PubMed] [Google Scholar]
- Fiamingo F. G., Brill A. S., Hampton D. A., Thorkildsen R. Energy distributions at the high-spin ferric sites in myoglobin crystals. Biophys J. 1989 Jan;55(1):67–77. doi: 10.1016/S0006-3495(89)82781-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H. W. Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta. 1991 Jul 30;1084(3):221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Kemal C., Louis-Flamberg P., Krupinski-Olsen R., Shorter A. L. Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity. Biochemistry. 1987 Nov 3;26(22):7064–7072. doi: 10.1021/bi00396a031. [DOI] [PubMed] [Google Scholar]
- Pistorius E. K., Axelrod B. Iron, an essential component of lipoxygenase. J Biol Chem. 1974 May 25;249(10):3183–3186. [PubMed] [Google Scholar]
- Pistorius E. K., Axelrod B., Palmer G. Evidence for participation of iron in lipoxygenase reaction from optical and electron spin resonance studies. J Biol Chem. 1976 Nov 25;251(22):7144–7148. [PubMed] [Google Scholar]
- Ramadoss C. S., Axelrod B. High-performance liquid chromatographic separation of lipoxygenase isozymes in crude soybean extracts. Anal Biochem. 1982 Nov 15;127(1):25–31. doi: 10.1016/0003-2697(82)90139-7. [DOI] [PubMed] [Google Scholar]
- Schilstra M. J., Veldink G. A., Verhagen J., Vliegenthart J. F. Effect of lipid hydroperoxide on lipoxygenase kinetics. Biochemistry. 1992 Aug 25;31(33):7692–7699. doi: 10.1021/bi00148a033. [DOI] [PubMed] [Google Scholar]
- Slappendel S., Malmström B. G., Petersson L., Ehrenberg A., Veldink G. A., Vliegenthart J. F. On the spin and valence state of iron in native soybean lipoxygenase-1. Biochem Biophys Res Commun. 1982 Sep 30;108(2):673–677. doi: 10.1016/0006-291x(82)90882-8. [DOI] [PubMed] [Google Scholar]
- Slappendel S., Veldink G. A., Vliegenthart J. F., Aasa R., Malmström B. G. EPR spectroscopy of soybean lipoxygenase-1. Description and quantification of the high-spin fe(III) signals. Biochim Biophys Acta. 1981 Jan 30;667(1):77–86. doi: 10.1016/0005-2795(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Slappendel S., Veldink G. A., Vliegenthart J. F., Aasa R., Malmström B. G. EPR spectroscopy of soybean lipoxygenase-1. Determination of the zero-field splitting constants of high-spin Fe(III) signals from temperature and microwave frequency dependence. Biochim Biophys Acta. 1980 Jul 24;624(1):30–39. doi: 10.1016/0005-2795(80)90222-6. [DOI] [PubMed] [Google Scholar]
- Sloane D. L., Browner M. F., Dauter Z., Wilson K., Fletterick R. J., Sigal E. Purification and crystallization of 15-lipoxygenase from rabbit reticulocytes. Biochem Biophys Res Commun. 1990 Dec 14;173(2):507–513. doi: 10.1016/s0006-291x(05)80063-4. [DOI] [PubMed] [Google Scholar]
- Spaapen L. J., Veldink G. A., Liefkens T. J., Vliegenthart J. F., Kay C. M. Circular dichroism of lipoxygenase-1 from soybeans. Biochim Biophys Acta. 1979 Aug 30;574(2):301–311. doi: 10.1016/0005-2760(79)90011-0. [DOI] [PubMed] [Google Scholar]
- Stallings W. C., Kroa B. A., Carroll R. T., Metzger A. L., Funk M. O. Crystallization and preliminary X-ray characterization of a soybean seed lipoxygenase. J Mol Biol. 1990 Feb 20;211(4):685–687. doi: 10.1016/0022-2836(90)90067-V. [DOI] [PubMed] [Google Scholar]
- Steczko J., Muchmore C. R., Smith J. L., Axelrod B. Crystallization and preliminary X-ray investigation of lipoxygenase 1 from soybeans. J Biol Chem. 1990 Jul 5;265(19):11352–11354. [PubMed] [Google Scholar]
- Van der Zee J., Eling T. E., Mason R. P. Formation of free radical metabolites in the reaction between soybean lipoxygenase and its inhibitors. An ESR study. Biochemistry. 1989 Oct 17;28(21):8363–8367. doi: 10.1021/bi00447a015. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Gaffney B. J. Determination of relative spin concentration in some high-spin ferric proteins using E/D-distribution in electron paramagnetic resonance simulations. Biophys J. 1987 Jan;51(1):55–67. doi: 10.1016/S0006-3495(87)83311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J. J., Veldink G. A., Vliegenthart J. F., Boldingh J., Wever R., van Gelder B. F. Demonstration by EPR spectroscopy of the functional role of iron in soybean lipoxygenase-1. Biochim Biophys Acta. 1975 Jan 23;377(1):71–79. doi: 10.1016/0005-2744(75)90287-9. [DOI] [PubMed] [Google Scholar]


