Abstract
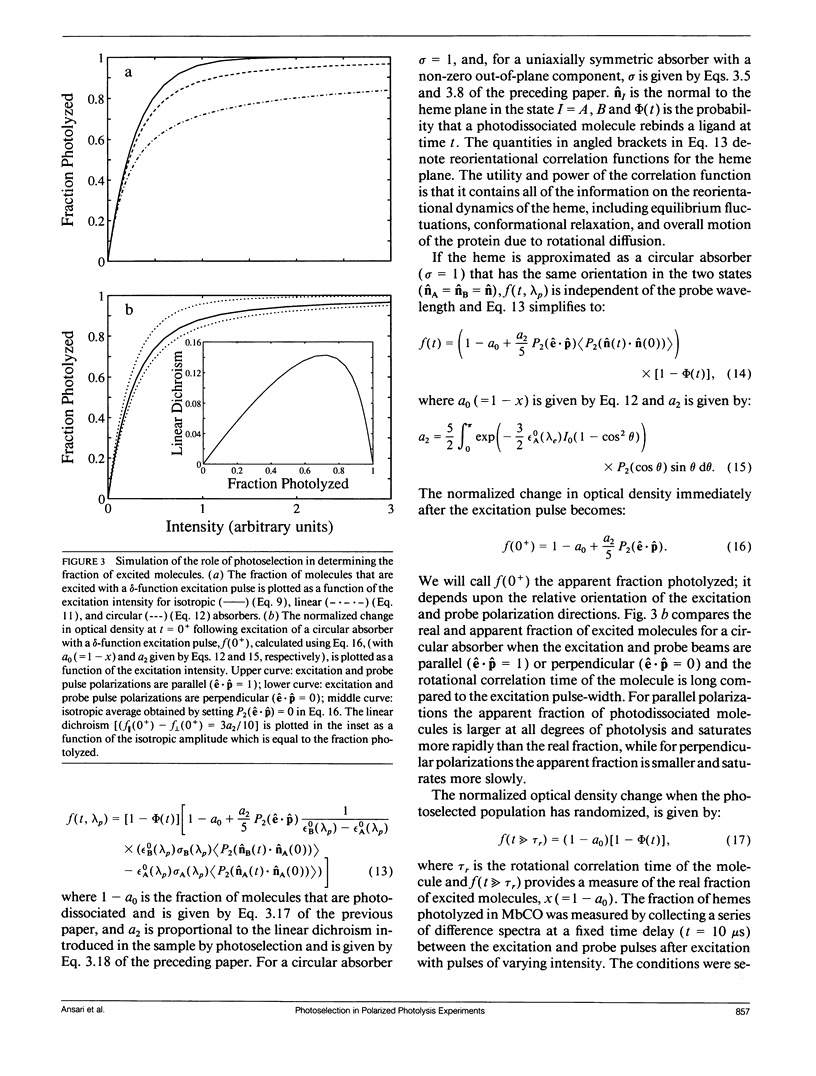
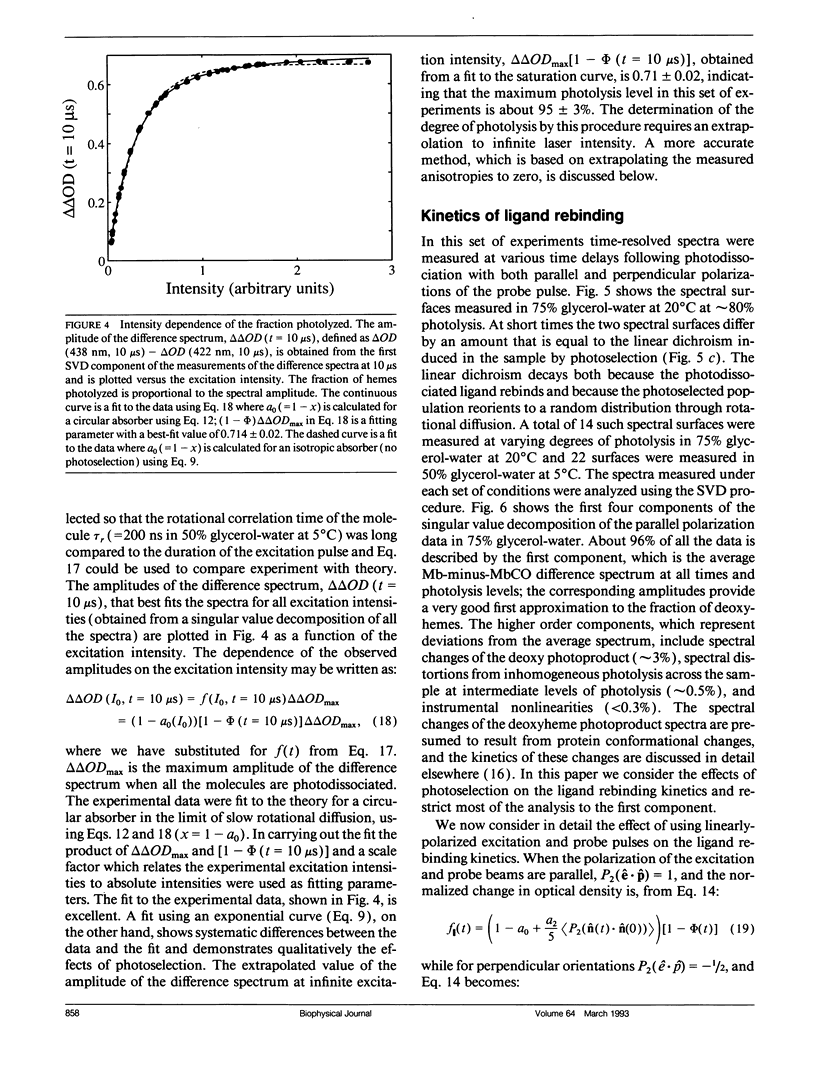
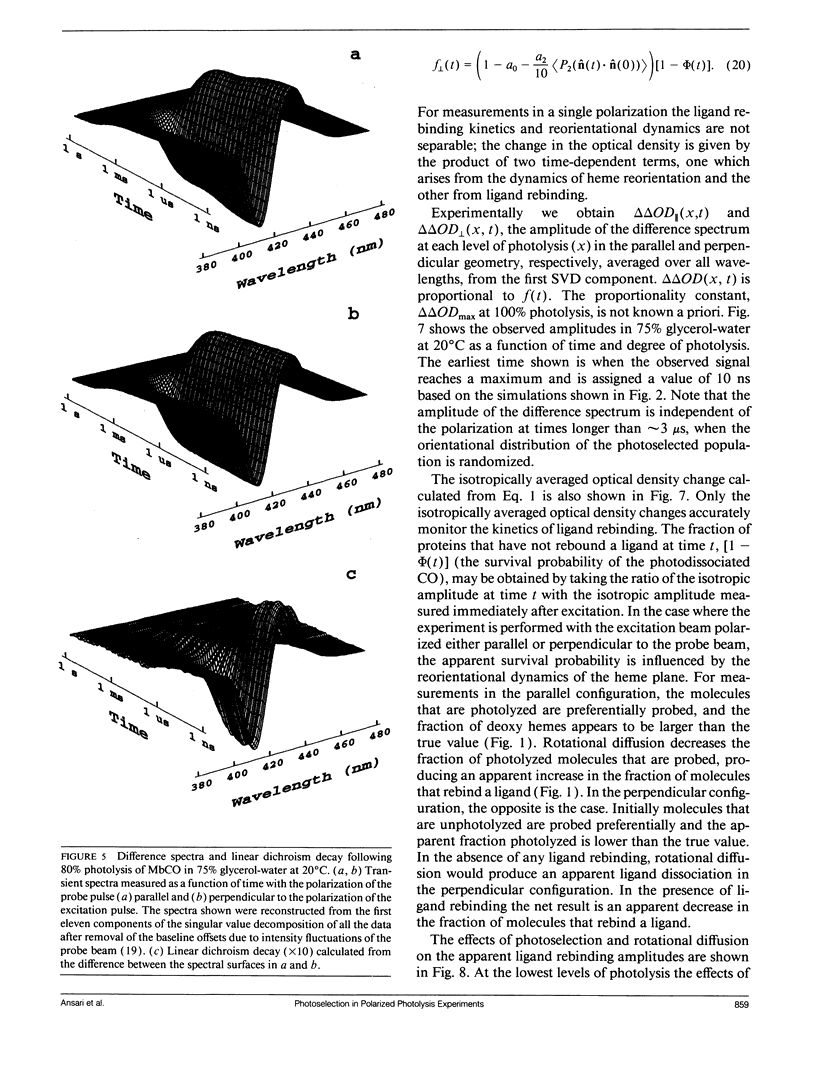
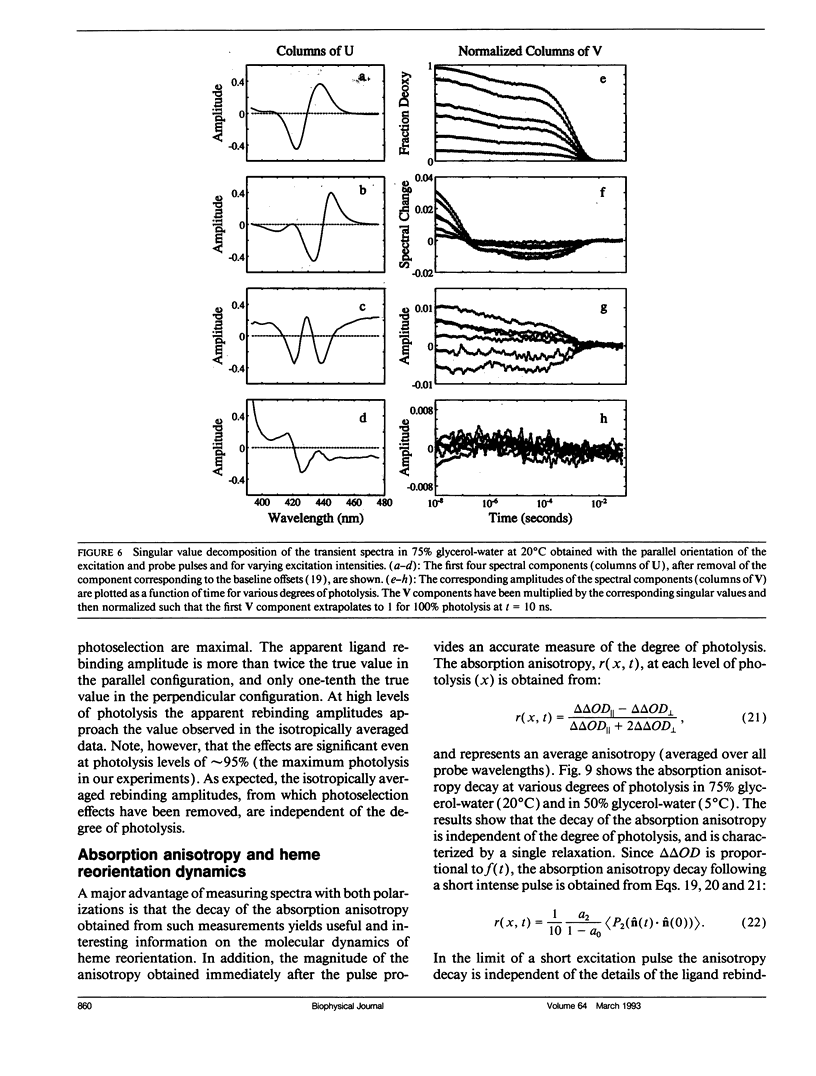
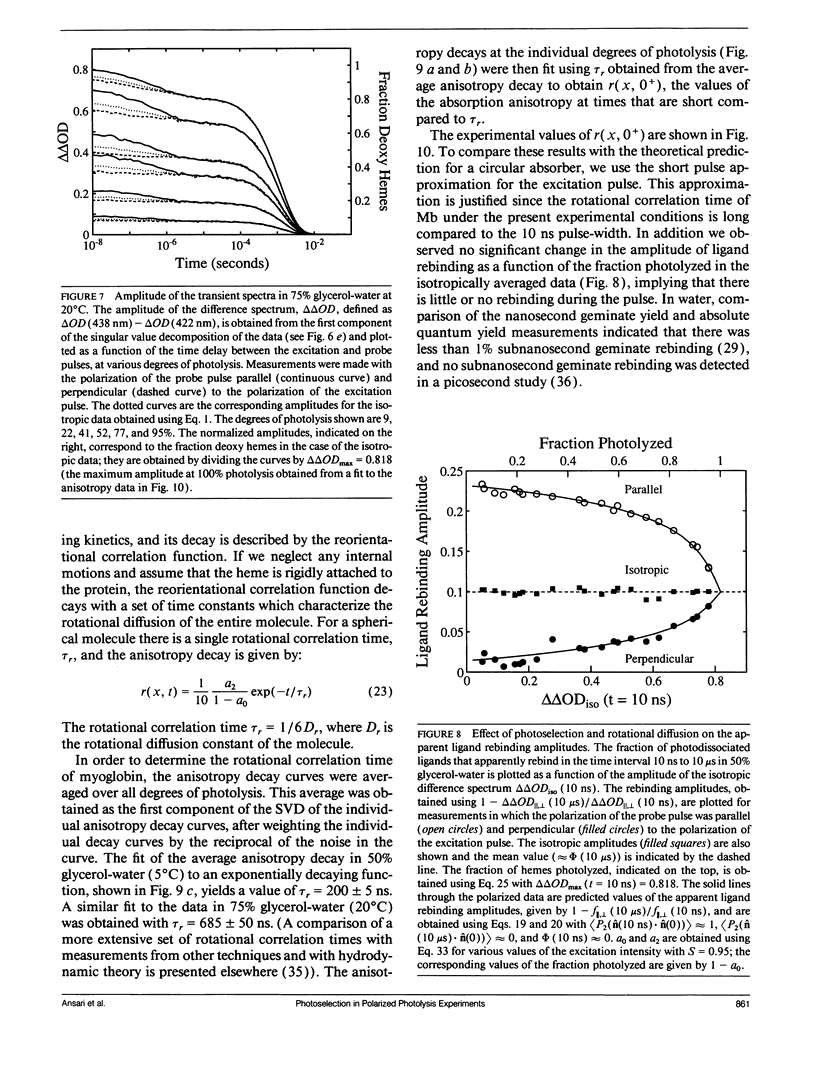
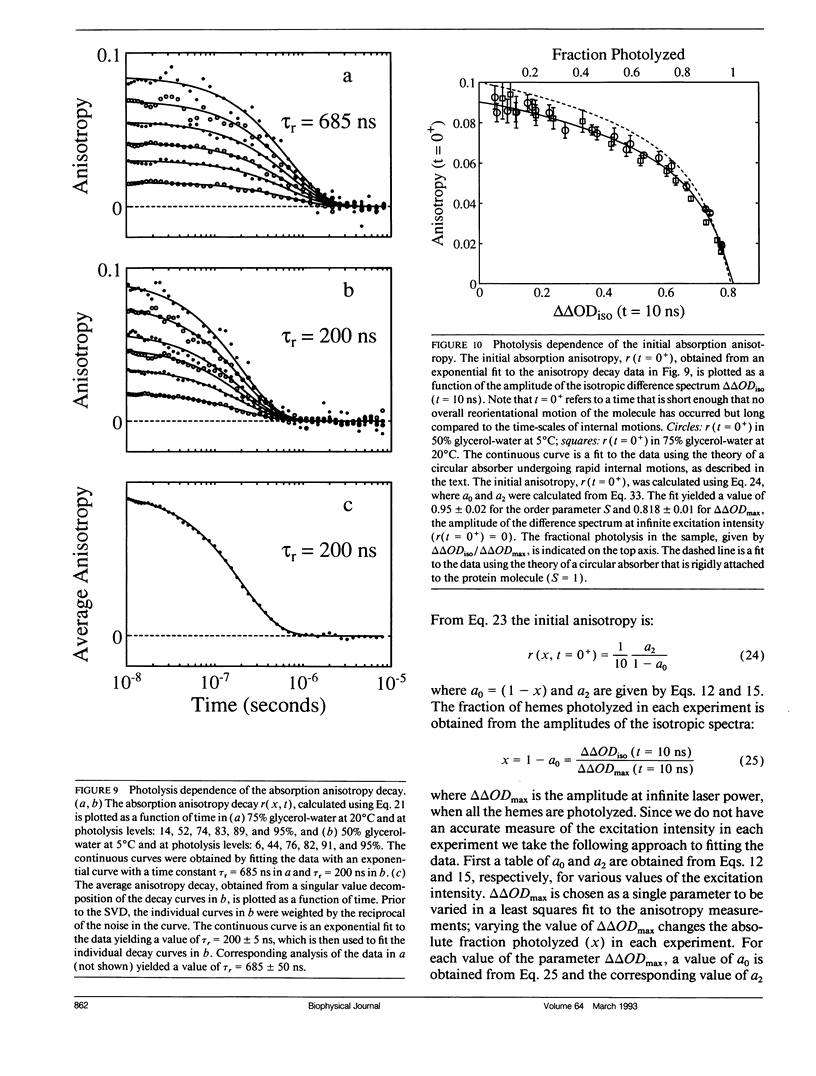
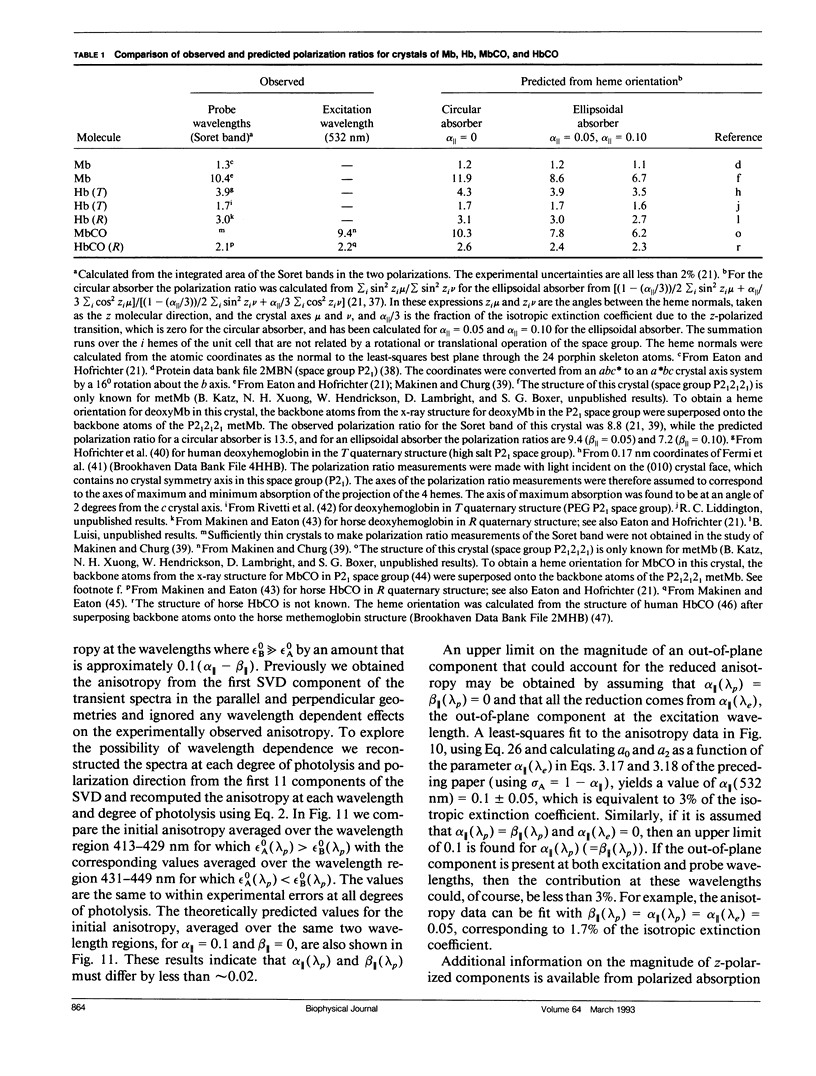
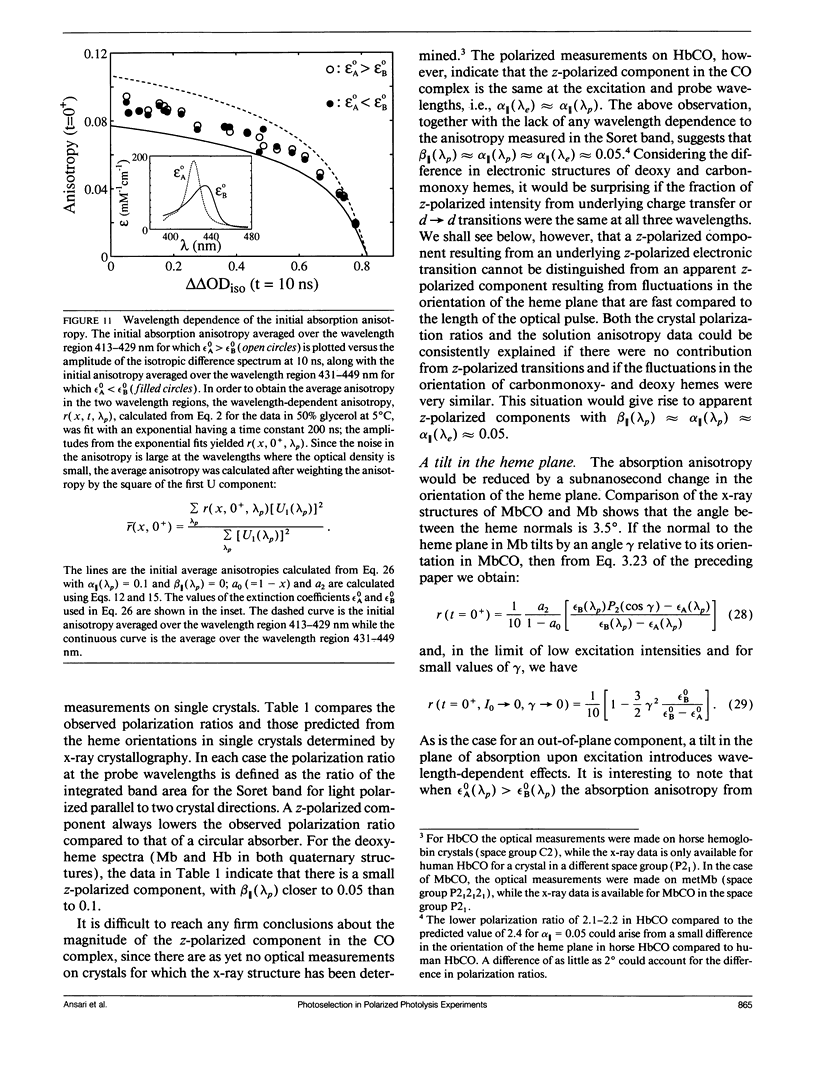
Polarized photolysis experiments have been performed on the carbon monoxide complex of myoglobin to assess the effects of photoselection on the kinetics of ligand rebinding and to investigate the reorientational dynamics of the heme plane. The results are analyzed in terms of the optical theory developed in the preceding paper by Ansari and Szabo. Changes in optical density arising from rotational diffusion of the photoselected population produce large deviations from the true geminate ligand rebinding curves if measurements are made with only a single polarization. The apparent ligand rebinding curves are significantly distorted even at photolysis levels greater than 90%. These deviations are eliminated by obtaining isotropically-averaged optical densities from measurements using both parallel and perpendicular polarizations of the probe pulse. These experiments also yield the optical anisotropy, which gives a novel method for accurately determining the degree of photolysis, as well as important information on the reorientational dynamics of the heme plane. The correlation time for the overall rotational diffusion of the molecule is obtained from the decay of the anisotropy. The anisotropy prior to rotational diffusion is lower than that predicted for a rigidly attached, perfectly circular absorber, corresponding to an apparent order parameter of S = 0.95 +/- 0.02. Polarized absorption data on single crystals suggest that the decreased anisotropy results more from internal motions of the heme plane which take place on time scales shorter than the duration of the laser pulse (10 ns) than from out-of-plane polarized transitions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinrud P. A., Han C., Hochstrasser R. M. Direct observations of ligand dynamics in hemoglobin by subpicosecond infrared spectroscopy. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8387–8391. doi: 10.1073/pnas.86.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992 Jun 26;256(5065):1796–1798. doi: 10.1126/science.1615323. [DOI] [PubMed] [Google Scholar]
- Ansari A., Szabo A. Theory of photoselection by intense light pulses. Influence of reorientational dynamics and chemical kinetics on absorbance measurements. Biophys J. 1993 Mar;64(3):838–851. doi: 10.1016/S0006-3495(93)81445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda Z., Dodson G., Emsley P., Harris D., Nagai K., Perutz M., Renaud J. P., Reynaud J. P. Stereochemistry of carbon monoxide binding to normal human adult and Cowtown haemoglobins. J Mol Biol. 1990 Feb 5;211(3):515–519. doi: 10.1016/0022-2836(90)90262-k. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Henry E. R., Hofrichter J. Application of linear free energy relations to protein conformational changes: the quaternary structural change of hemoglobin. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4472–4475. doi: 10.1073/pnas.88.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Single-crystal spectra of ferrimyoglobin complexes in polarized light. J Chem Phys. 1968 Aug 1;49(3):985–995. doi: 10.1063/1.1670263. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- Greene B. I., Hochstrasser R. M., Weisman R. B., Eaton W. A. Spectroscopic studies of oxy- and carbonmonoxyhemoglobin after pulsed optical excitation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Levitt M., Eaton W. A. Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2034–2038. doi: 10.1073/pnas.82.7.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R. Molecular dynamics simulations of heme reorientational motions in myoglobin. Biophys J. 1993 Mar;64(3):869–885. doi: 10.1016/S0006-3495(93)81447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Sommer J. H., Hofrichter J., Eaton W. A. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol. 1983 May 25;166(3):443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Eaton W. A. Linear dichroism of biological chromophores. Annu Rev Biophys Bioeng. 1976;5:511–560. doi: 10.1146/annurev.bb.05.060176.002455. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Hendricker D. G., Eaton W. A. Structure of hemoglobin S fibers: optical determination of the molecular orientation in sickled erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3604–3608. doi: 10.1073/pnas.70.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Sommer J. H., Deutsch R., Ikeda-Saito M., Yonetani T., Eaton W. A. Nanosecond optical spectra of iron-cobalt hybrid hemoglobins: geminate recombination, conformational changes, and intersubunit communication. Biochemistry. 1985 May 21;24(11):2667–2679. doi: 10.1021/bi00332a012. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Szabo A., Murray L. P., Ansari A., Jones C. M., Coletta M., Falcioni G., Brunori M., Eaton W. A. Dynamics of the quaternary conformational change in trout hemoglobin. Biochemistry. 1991 Jul 2;30(26):6583–6598. doi: 10.1021/bi00240a031. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. M., Dalickas G. A., Eaton W. A., Hochstrasser R. M. Picosecond transient absorption study of photodissociated carboxy hemoglobin and myoglobin. Biophys J. 1988 Sep;54(3):545–549. doi: 10.1016/S0006-3495(88)82987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Ansari A., Henry E. R., Christoph G. W., Hofrichter J., Eaton W. A. Speed of intersubunit communication in proteins. Biochemistry. 1992 Jul 28;31(29):6692–6702. doi: 10.1021/bi00144a008. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Lewis J. W., Kliger D. S. Rotational diffusion effects on absorbance measurements: limitations to the magic-angle approach. Photochem Photobiol. 1991 Dec;54(6):963–968. doi: 10.1111/j.1751-1097.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Lipari G., Szabo A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys J. 1980 Jun;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Optically detected conformational changes in haemoglobin single crystals. Nature. 1974 Jan 4;247(5435):62–64. doi: 10.1038/247062a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Polarized single crystal absorption spectra of carboxy- and oxyhemoglobin. Ann N Y Acad Sci. 1973;206:210–222. doi: 10.1111/j.1749-6632.1973.tb43213.x. [DOI] [PubMed] [Google Scholar]
- Marden M. C., Hazard E. S., Gibson Q. H. Testing the two-state model: anomalous effector binding to human hemoglobin. Biochemistry. 1986 Nov 18;25(23):7591–7596. doi: 10.1021/bi00371a049. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Ikeda-Saito M., Kitagishi K., Yonetani T., Eaton W. A. The effect of quaternary structure on the kinetics of conformational changes and nanosecond geminate rebinding of carbon monoxide to hemoglobin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2151–2155. doi: 10.1073/pnas.85.7.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]


