Abstract
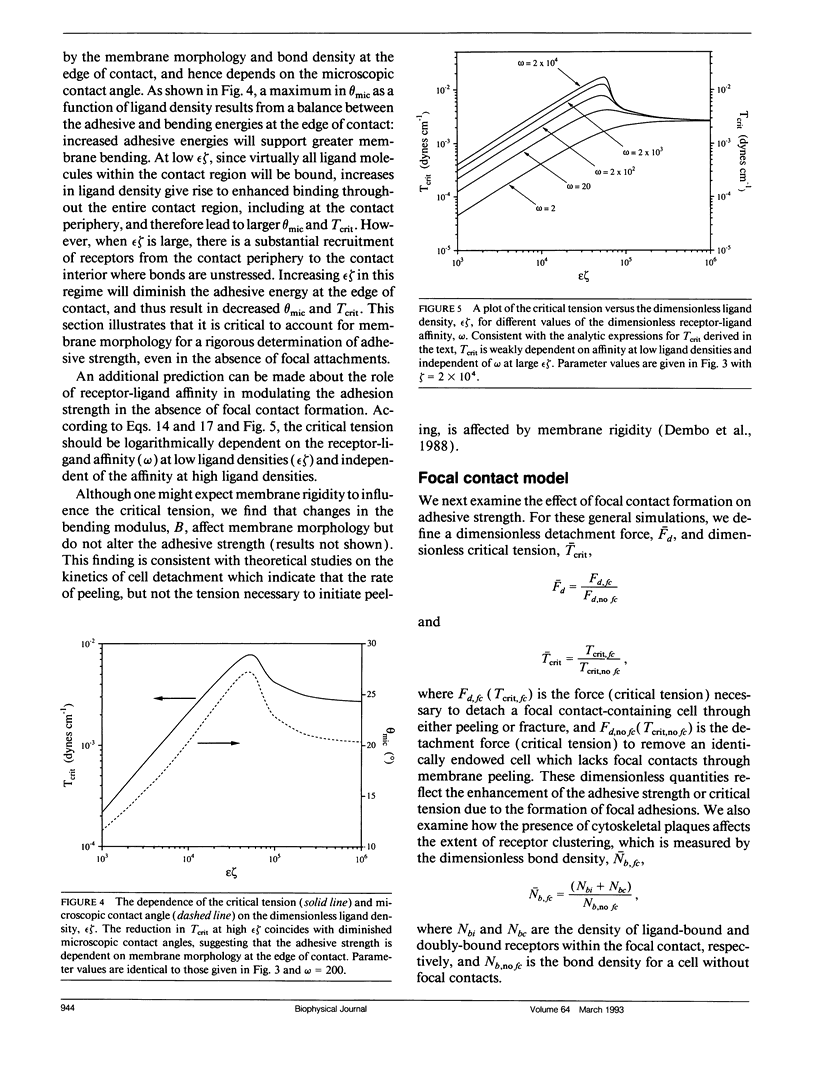
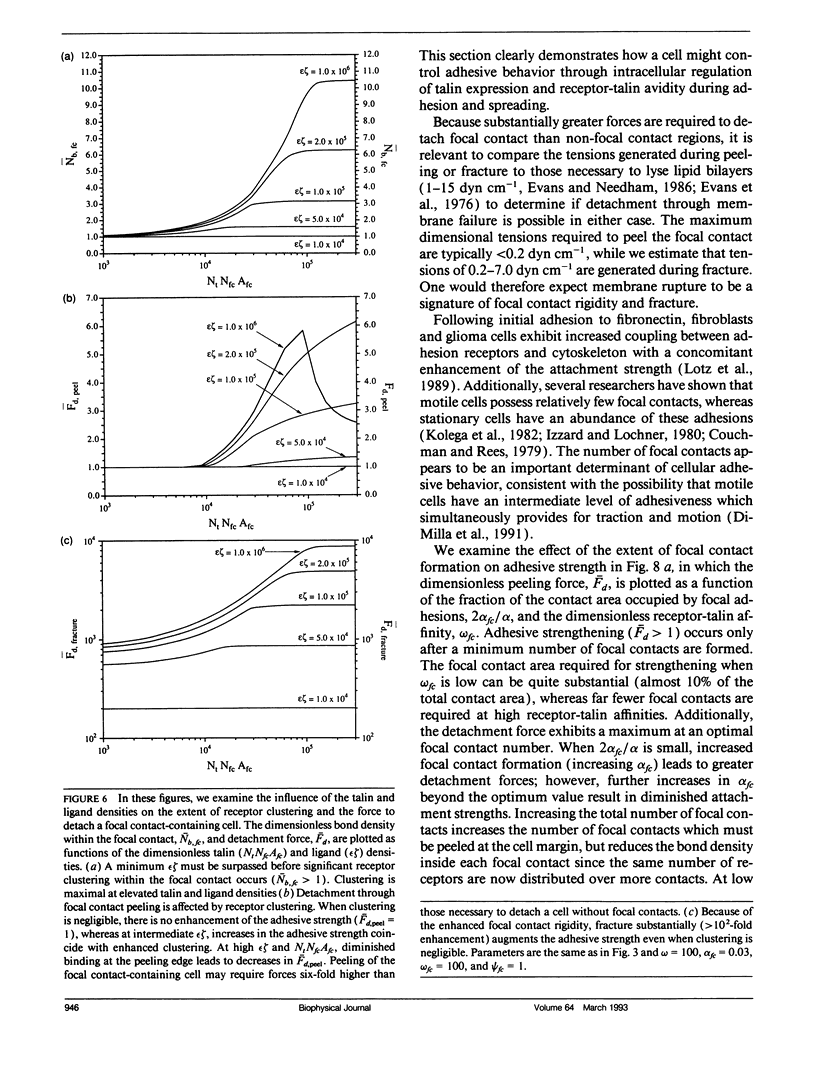
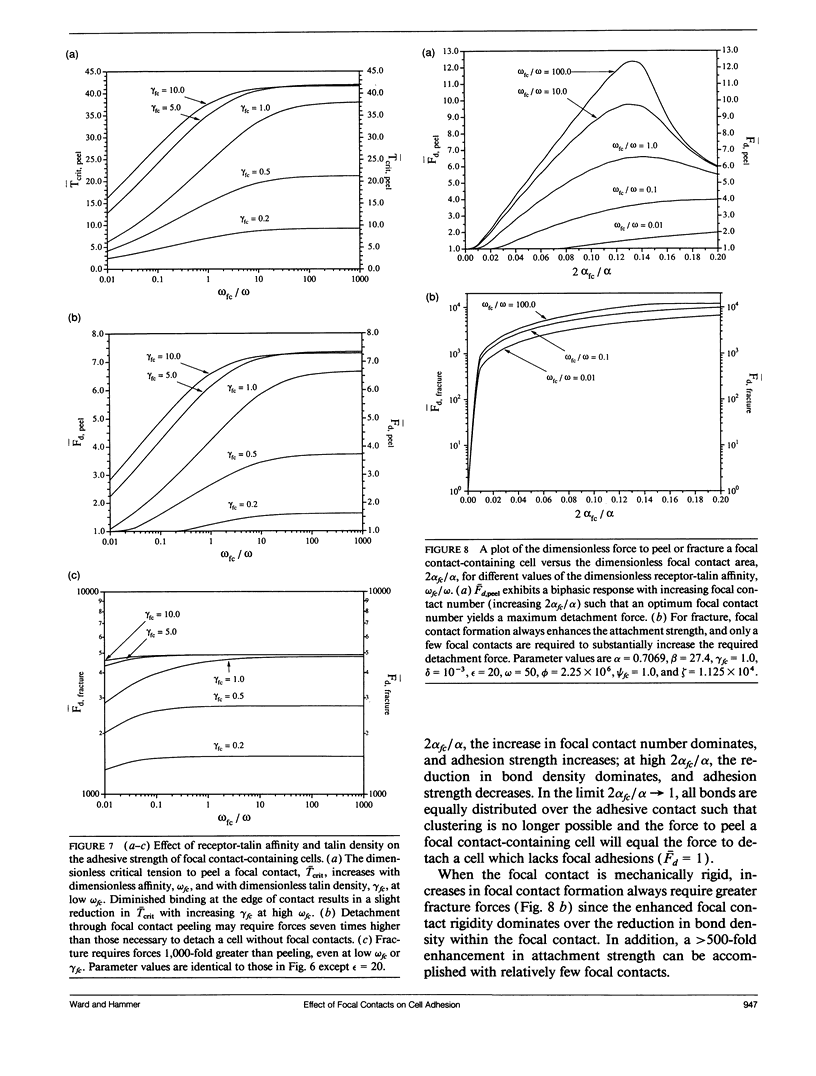
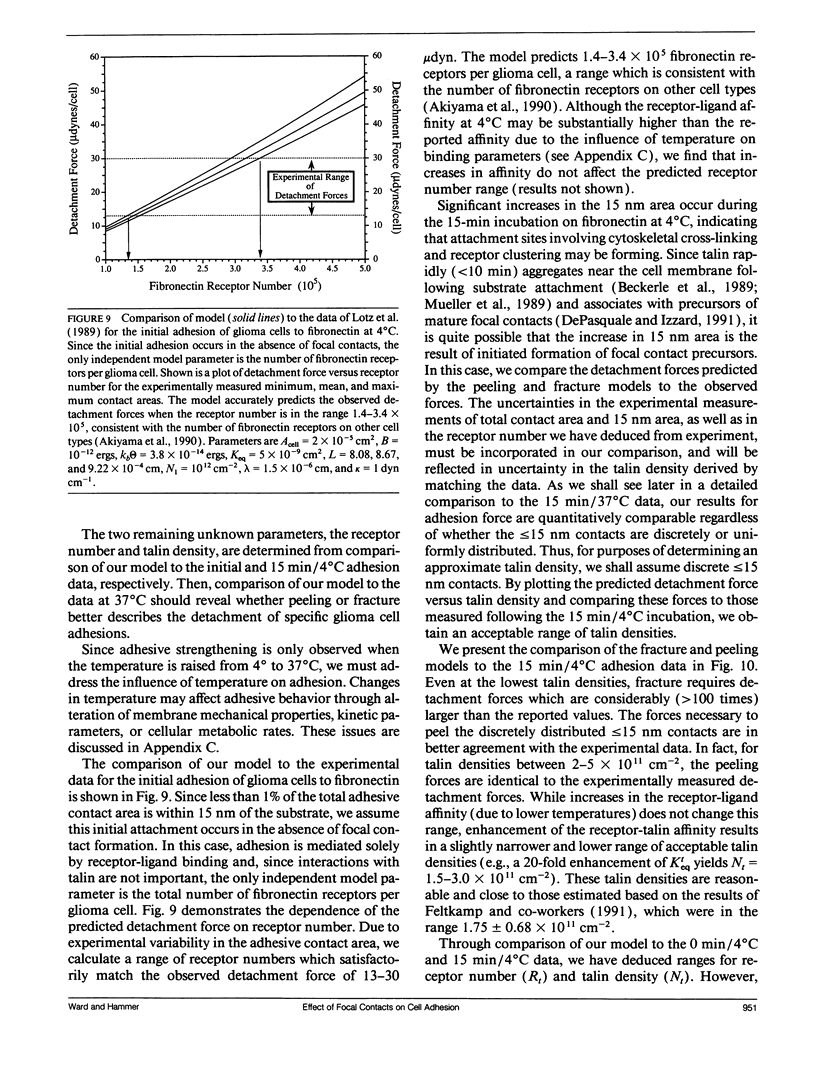
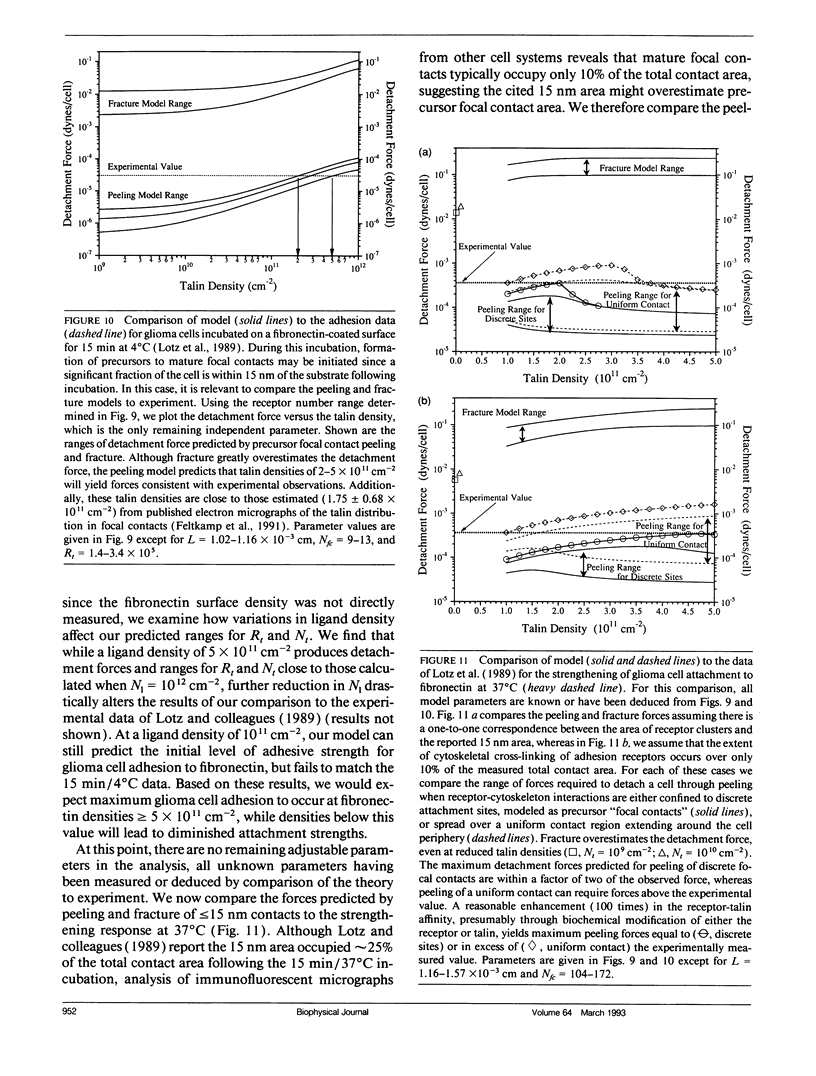
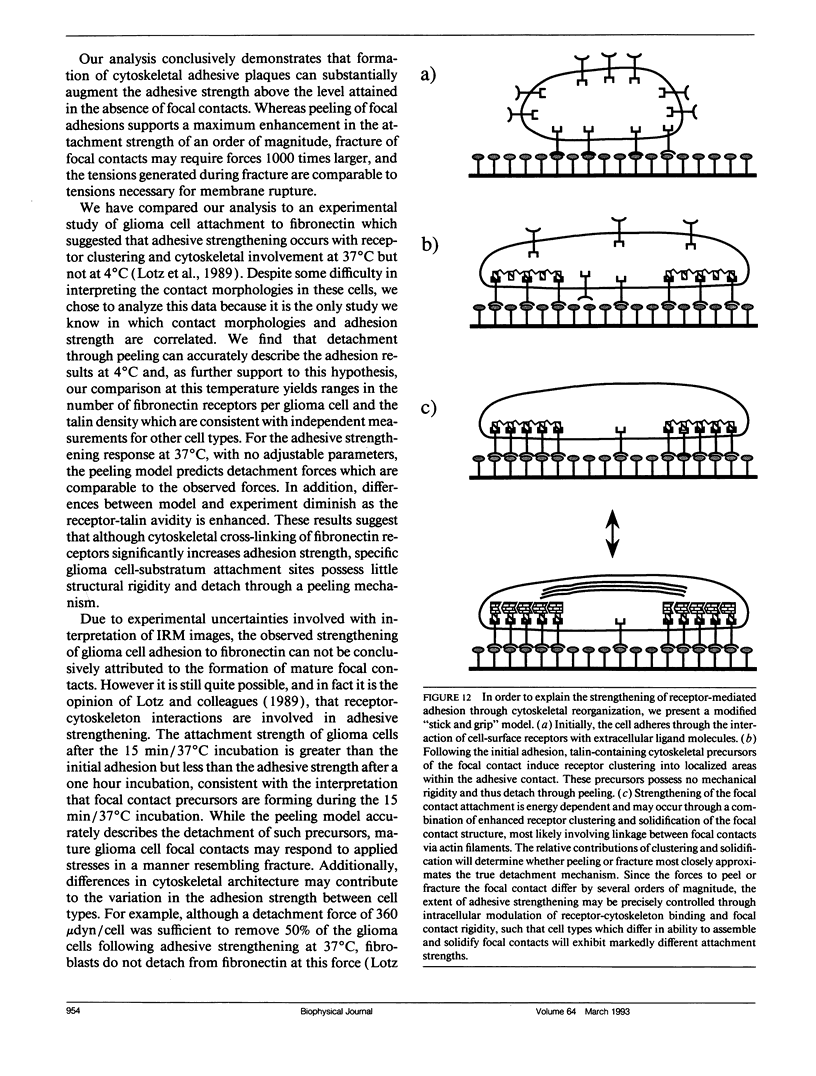
For many cell types, growth, differentiation, and motility are dependent on receptor-mediated adhesion to ligand-coated surfaces. Focal contacts are strong, specialized, adhesive connections between cell and substrate in which receptors aggregate and connect extracellular ligand to intracellular cytoskeletal molecules. In this paper, we present a mathematical model to examine how focal contact formation affects cellular adhesive strength. To calculate adhesive strength with and without focal contacts, we use a one-dimensional tape peeling analysis to determine the critical tension necessary to peel the membrane. Receptor-ligand bonds are modeled as adhesive springs. In the absence of focal contacts, we derive analytic expressions for the critical tension at low and high ligand densities and show how membrane morphology affects adhesion. Then, focal contacts are modeled as cytoplasmic nucleation centers which bind adhesion receptors. The extent of adhesive strengthening upon focal contact formation depends on the elastic rigidity of the cytoskeletal connections, which determines the structural integrity of the focal contact itself. We consider two limits to this elasticity, very weak and rigid. Rigid cytoskeletal connections give much greater attachment strengths. The dependence of attachment strength on measurable model parameters is quite different in these two limits, which suggests focal contact structure might be deduced from properly performed adhesion experiments. Finally, we compare our model to the adhesive strengthening response reported for glioma cell adhesion to fibronectin (Lotz et al., 1989. J. Cell Biol. 109:1795-1805). Our model successfully predicts the observed detachment forces at 4 degrees C and yields values for the number of fibronectin receptors per glioma cell and the density of cytoskeletal connection molecules (talin) involved in receptor clusters which are consistent with measurements for other cell types. Comparison of the model with data at 37 degrees C suggests that while cytoskeletal cross-linking and clustering of fibronectin receptors significantly increases adhesion strength, specific glioma cell-substratum attachment sites possess little mechanical rigidity and detach through a peeling mechanism, consistent with the view that these sites of < or = 15 nm cell-substrate separation are precursors to fully formed, elastically rigid focal contacts.
Full text
PDF

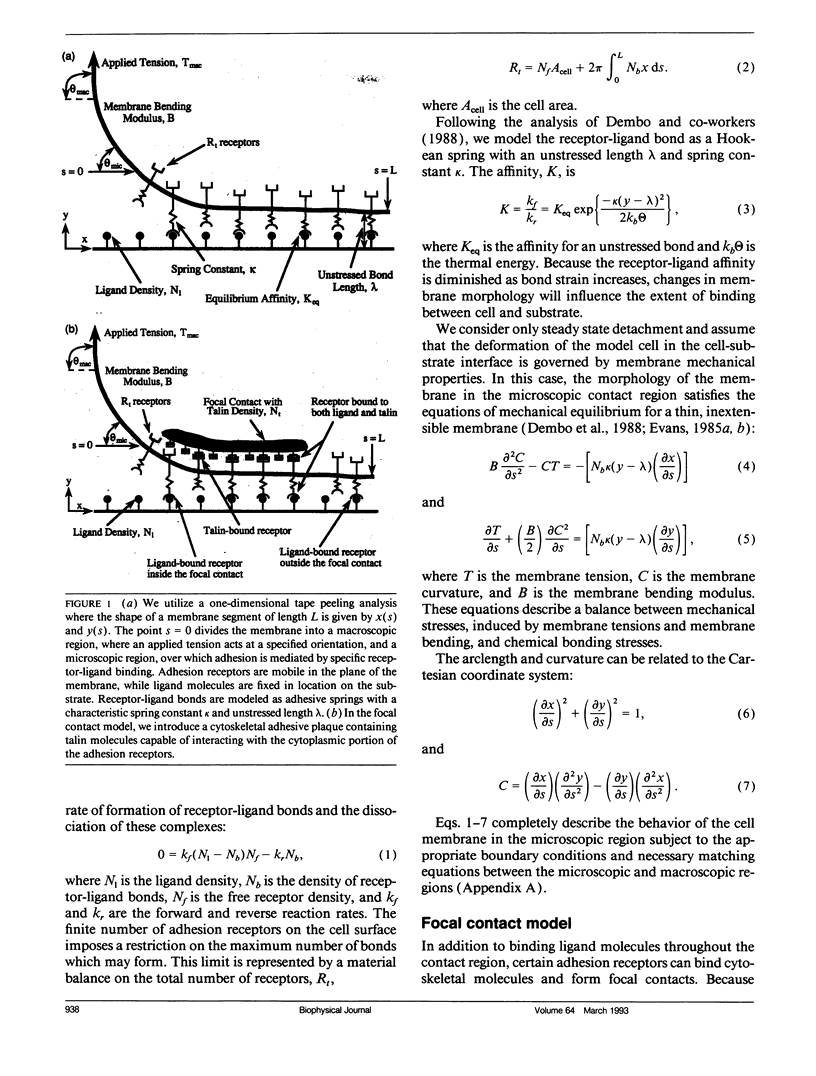


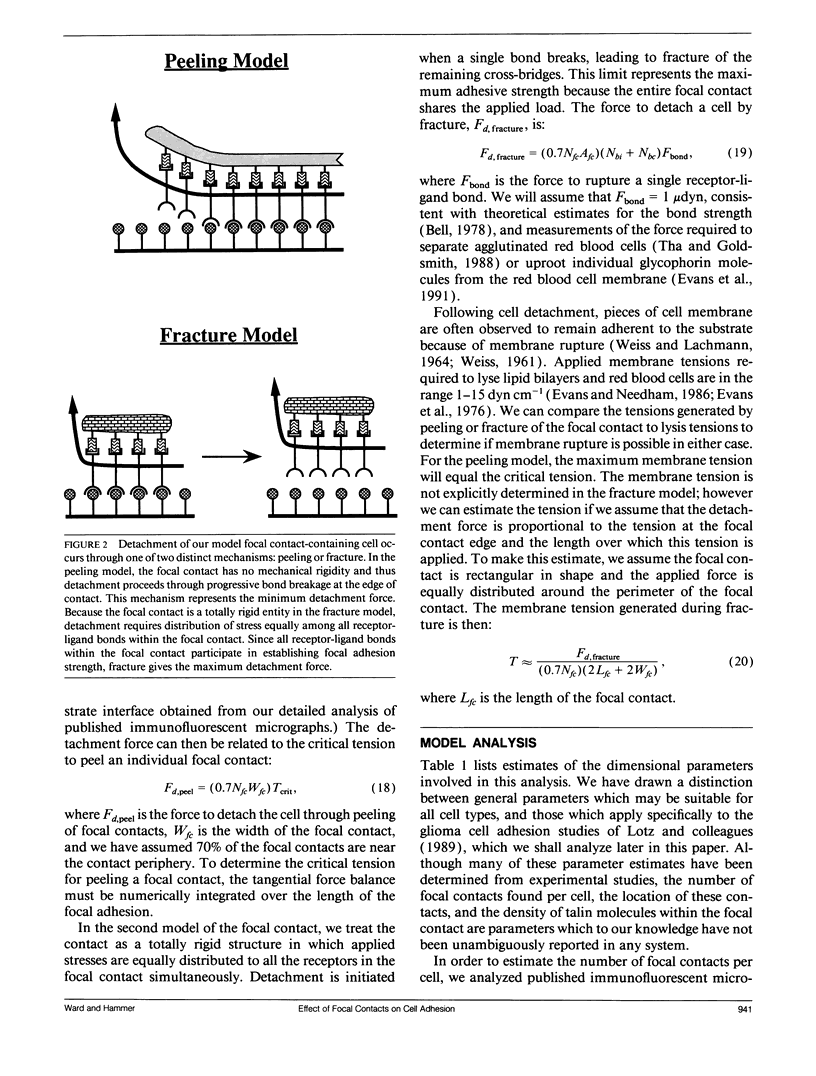
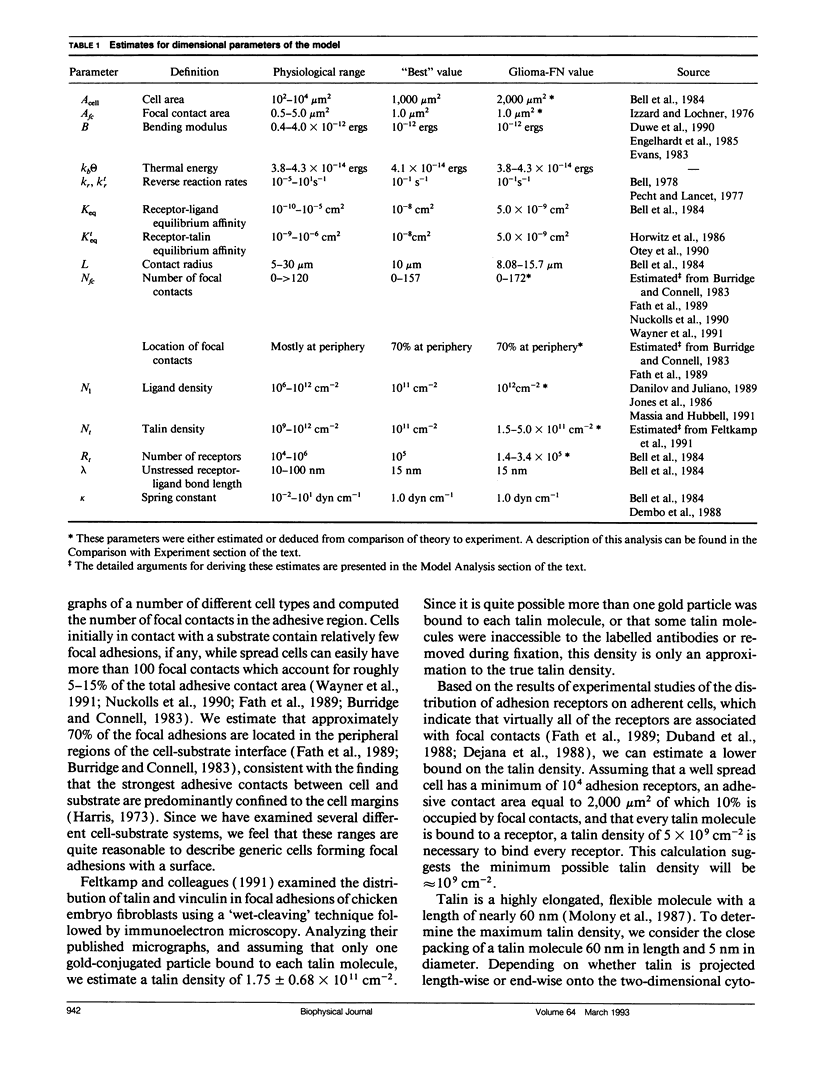
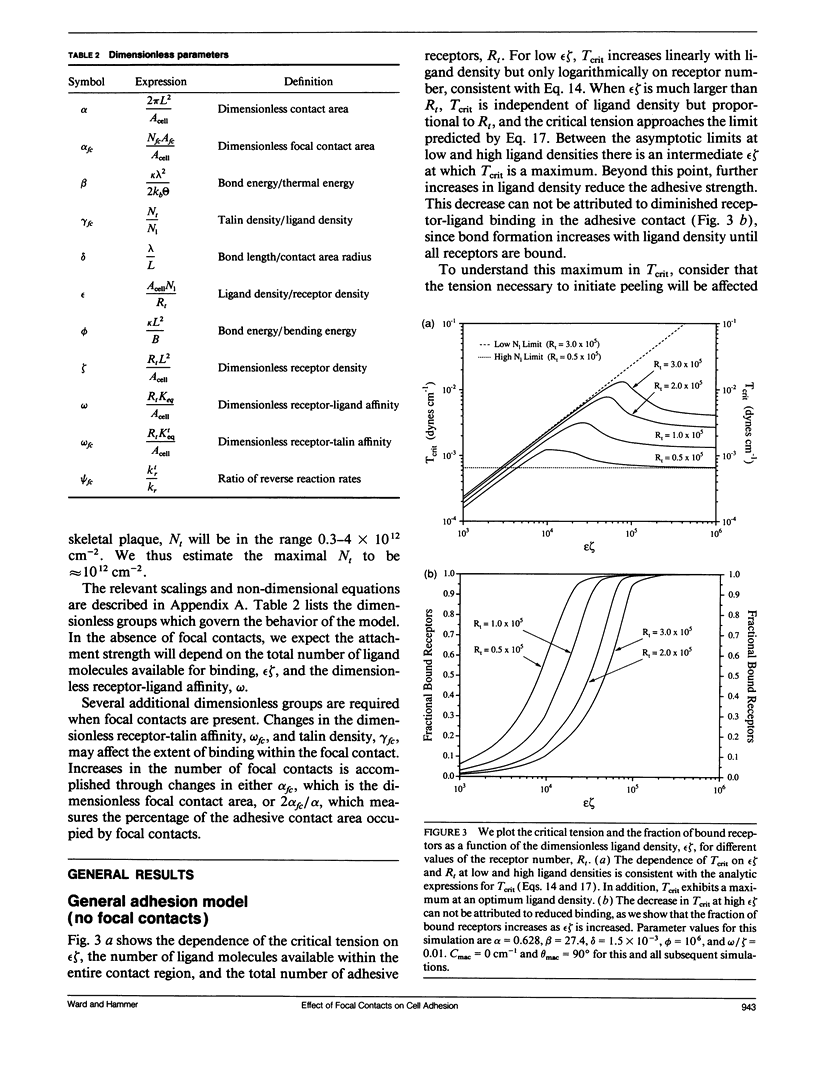










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971 Aug;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Nagata K., Yamada K. M. Cell surface receptors for extracellular matrix components. Biochim Biophys Acta. 1990 Feb 28;1031(1):91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada K. M. Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional biological assays for the purified cell-binding domain of fibronectin. J Biol Chem. 1985 Sep 5;260(19):10402–10405. [PubMed] [Google Scholar]
- Aumailley M., Gurrath M., Müller G., Calvete J., Timpl R., Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991 Oct 7;291(1):50–54. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Thompson N. L., Burghardt T. P. Total internal inflection fluorescent microscopy. J Microsc. 1983 Jan;129(Pt 1):19–28. doi: 10.1111/j.1365-2818.1983.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C., Miller D. E., Bertagnolli M. E., Locke S. J. Activation-dependent redistribution of the adhesion plaque protein, talin, in intact human platelets. J Cell Biol. 1989 Dec;109(6 Pt 2):3333–3346. doi: 10.1083/jcb.109.6.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Robinson G. S., Bucher N. L., Farmer S. R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3(5-6):405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. An interaction between vinculin and talin. Nature. 1984 Apr 19;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Wang J., Hasegawa T., Yamada S. S., Yamada K. M. Regulation of fibronectin receptor distribution by transformation, exogenous fibronectin, and synthetic peptides. J Cell Biol. 1986 Nov;103(5):1649–1661. doi: 10.1083/jcb.103.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenberger D. A. Receptor-mediated adhesion phenomena. Model studies with the Radical-Flow Detachment Assay. Biophys J. 1990 Jul;58(1):107–125. doi: 10.1016/S0006-3495(90)82357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov Y. N., Juliano R. L. (Arg-Gly-Asp)n-albumin conjugates as a model substratum for integrin-mediated cell adhesion. Exp Cell Res. 1989 May;182(1):186–196. doi: 10.1016/0014-4827(89)90290-5. [DOI] [PubMed] [Google Scholar]
- DePasquale J. A., Izzard C. S. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J Cell Biol. 1991 Jun;113(6):1351–1359. doi: 10.1083/jcb.113.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Harlow F. Cell motion, contractile networks, and the physics of interpenetrating reactive flow. Biophys J. 1986 Jul;50(1):109–121. doi: 10.1016/S0006-3495(86)83444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- DiMilla P. A., Barbee K., Lauffenburger D. A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991 Jul;60(1):15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J. L., Nuckolls G. H., Ishihara A., Hasegawa T., Yamada K. M., Thiery J. P., Jacobson K. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988 Oct;107(4):1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys J. 1985 Jul;48(1):175–183. doi: 10.1016/S0006-3495(85)83770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. II. Discrete kinetically trapped molecular cross-bridges. Biophys J. 1985 Jul;48(1):185–192. doi: 10.1016/S0006-3495(85)83771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A. Detachment of agglutinin-bonded red blood cells. I. Forces to rupture molecular-point attachments. Biophys J. 1991 Apr;59(4):838–848. doi: 10.1016/S0006-3495(91)82296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Needham D. Giant vesicle bilayers composed of mixtures of lipids, cholesterol and polypeptides. Thermomechanical and (mutual) adherence properties. Faraday Discuss Chem Soc. 1986;(81):267–280. doi: 10.1039/dc9868100267. [DOI] [PubMed] [Google Scholar]
- Fath K. R., Edgell C. J., Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989 Jan;92(Pt 1):67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Feltkamp C. A., Pijnenburg M. A., Roos E. Organization of talin and vinculin in adhesion plaques of wet-cleaved chicken embryo fibroblasts. J Cell Sci. 1991 Nov;100(Pt 3):579–587. doi: 10.1242/jcs.100.3.579. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I., Bailey J. Topography of cell-glass apposition revealed by total internal reflection fluorescence of volume markers. J Cell Biol. 1985 Apr;100(4):1334–1338. doi: 10.1083/jcb.100.4.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Lauffenburger D. A. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987 Sep;52(3):475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. Location of cellular adhesions to solid substrata. Dev Biol. 1973 Nov;35(1):97–114. doi: 10.1016/0012-1606(73)90009-2. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Arumugham R. G., Tanzer M. L. Fibronectin glycosylation modulates fibroblast adhesion and spreading. J Cell Biol. 1986 Nov;103(5):1663–1670. doi: 10.1083/jcb.103.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J., Shure M. S., Chen W. T., Young N. D. Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci. 1982 Apr;54:23–34. doi: 10.1242/jcs.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Tajima M., Ueno Y., Giga-Hama Y., Ohba M. Effect of cyclic RGD peptide on cell adhesion and tumor metastasis. Biochem Biophys Res Commun. 1991 May 31;177(1):74–82. doi: 10.1016/0006-291x(91)91950-h. [DOI] [PubMed] [Google Scholar]
- Lotz M. M., Burdsal C. A., Erickson H. P., McClay D. R. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989 Oct;109(4 Pt 1):1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991 Sep;114(5):1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990 Jun;187(2):292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- Molony L., McCaslin D., Abernethy J., Paschal B., Burridge K. Properties of talin from chicken gizzard smooth muscle. J Biol Chem. 1987 Jun 5;262(16):7790–7795. [PubMed] [Google Scholar]
- Mueller S. C., Kelly T., Dai M. Z., Dai H. N., Chen W. T. Dynamic cytoskeleton-integrin associations induced by cell binding to immobilized fibronectin. J Cell Biol. 1989 Dec;109(6 Pt 2):3455–3464. doi: 10.1083/jcb.109.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckolls G. H., Turner C. E., Burridge K. Functional studies of the domains of talin. J Cell Biol. 1990 May;110(5):1635–1644. doi: 10.1083/jcb.110.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill C., Jordan P., Ireland G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell. 1986 Feb 14;44(3):489–496. doi: 10.1016/0092-8674(86)90470-8. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto J. J. Vinculin. Cell Motil Cytoskeleton. 1990;16(1):1–6. doi: 10.1002/cm.970160102. [DOI] [PubMed] [Google Scholar]
- Pecht I., Lancet D. Kinetics of antibody-hapten interactions. Mol Biol Biochem Biophys. 1977;24:306–338. doi: 10.1007/978-3-642-81117-3_9. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Tha S. P., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. III. Micromanipulation. Biophys J. 1988 May;53(5):677–687. doi: 10.1016/S0006-3495(88)83149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozeren A., Sung K. L., Chien S. Theoretical and experimental studies on cross-bridge migration during cell disaggregation. Biophys J. 1989 Mar;55(3):479–487. doi: 10.1016/S0006-3495(89)82841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Sung K. L., Sung L. A., Dustin M. L., Chan P. Y., Springer T. A., Chien S. Micromanipulation of adhesion of a Jurkat cell to a planar bilayer membrane containing lymphocyte function-associated antigen 3 molecules. J Cell Biol. 1992 Feb;116(4):997–1006. doi: 10.1083/jcb.116.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS L., LACHMANN P. J. THE ORIGIN OF AN ANTIGENIC ZONE SURROUNDING HELA CELLS CULTURED ON GLASS. Exp Cell Res. 1964 Oct;36:86–91. doi: 10.1016/0014-4827(64)90162-4. [DOI] [PubMed] [Google Scholar]
- WEISS L. Studies on cellular adhesion in tissue culture. IV. The alteration of substrata by cell surfaces. Exp Cell Res. 1961 Dec;25:504–517. doi: 10.1016/0014-4827(61)90186-0. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Jordan P. W., O'Neill C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]


