Abstract
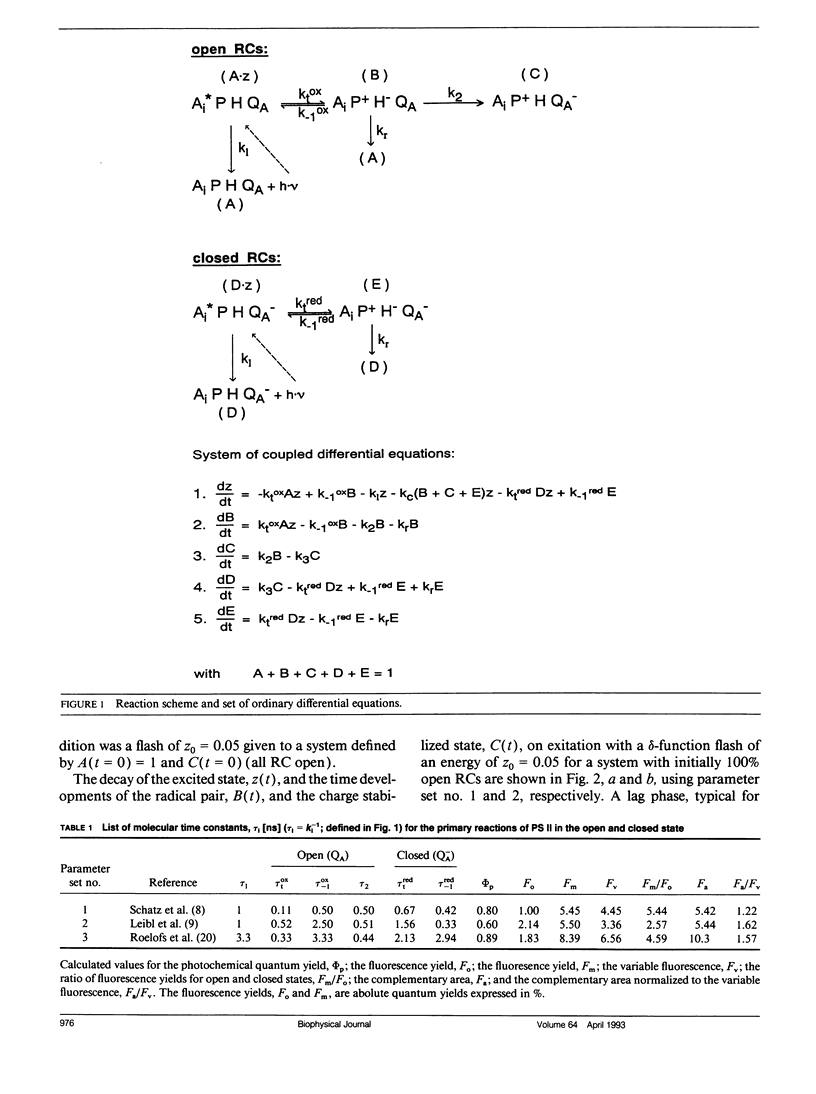
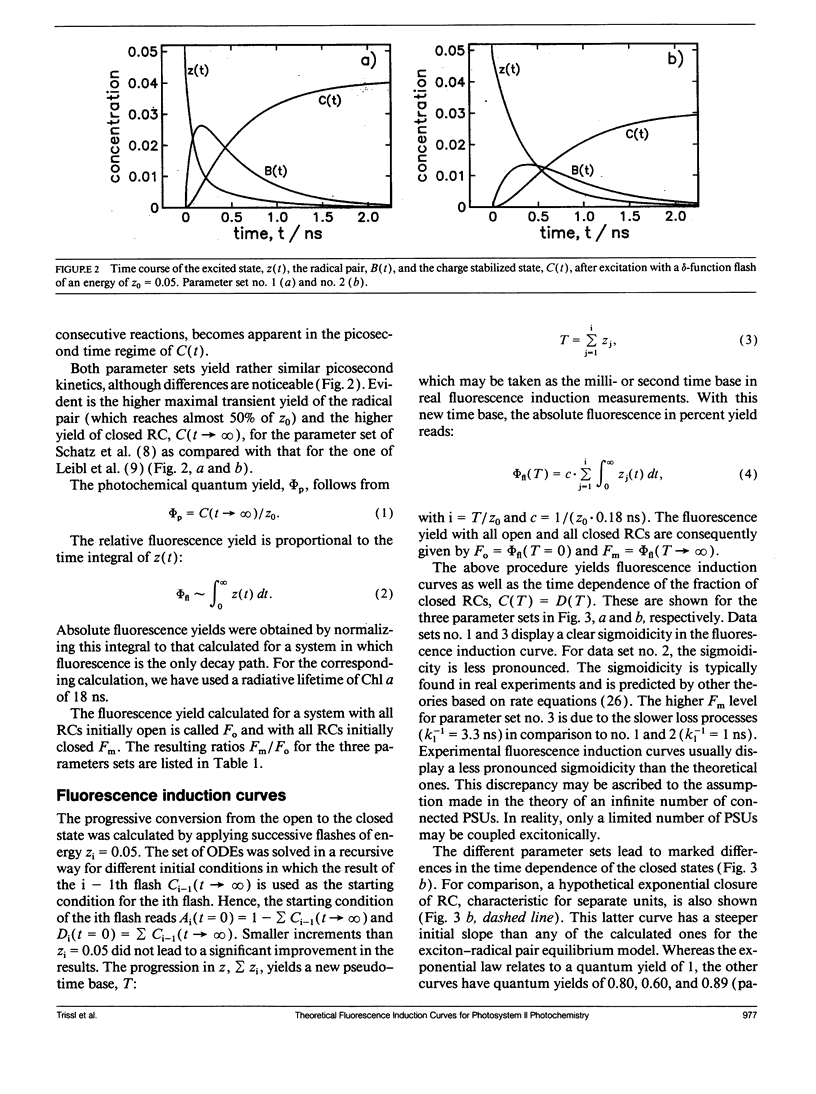
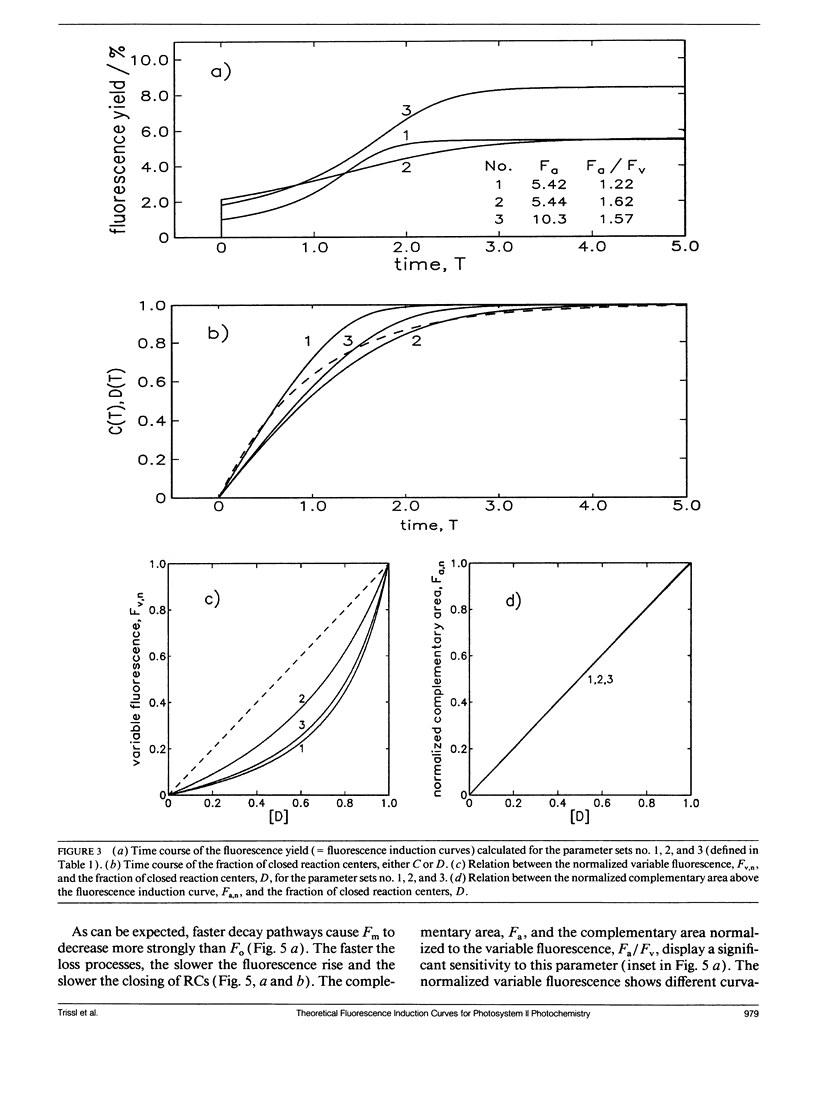
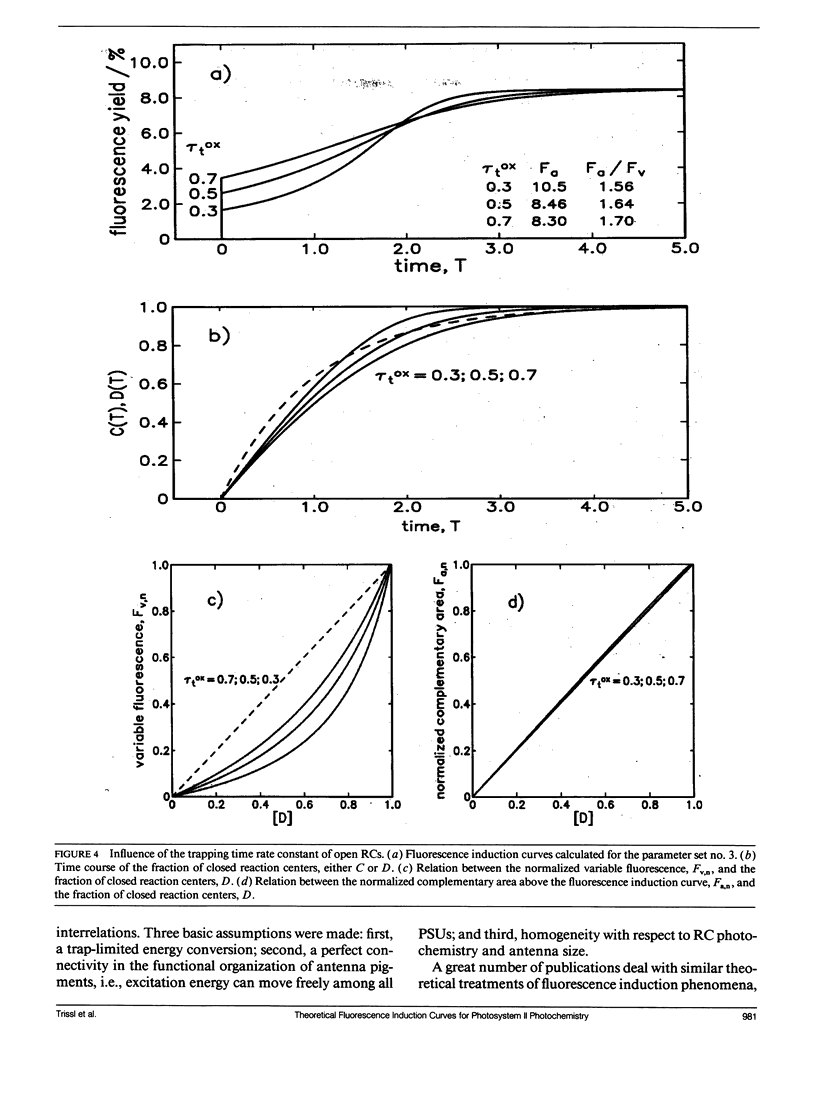
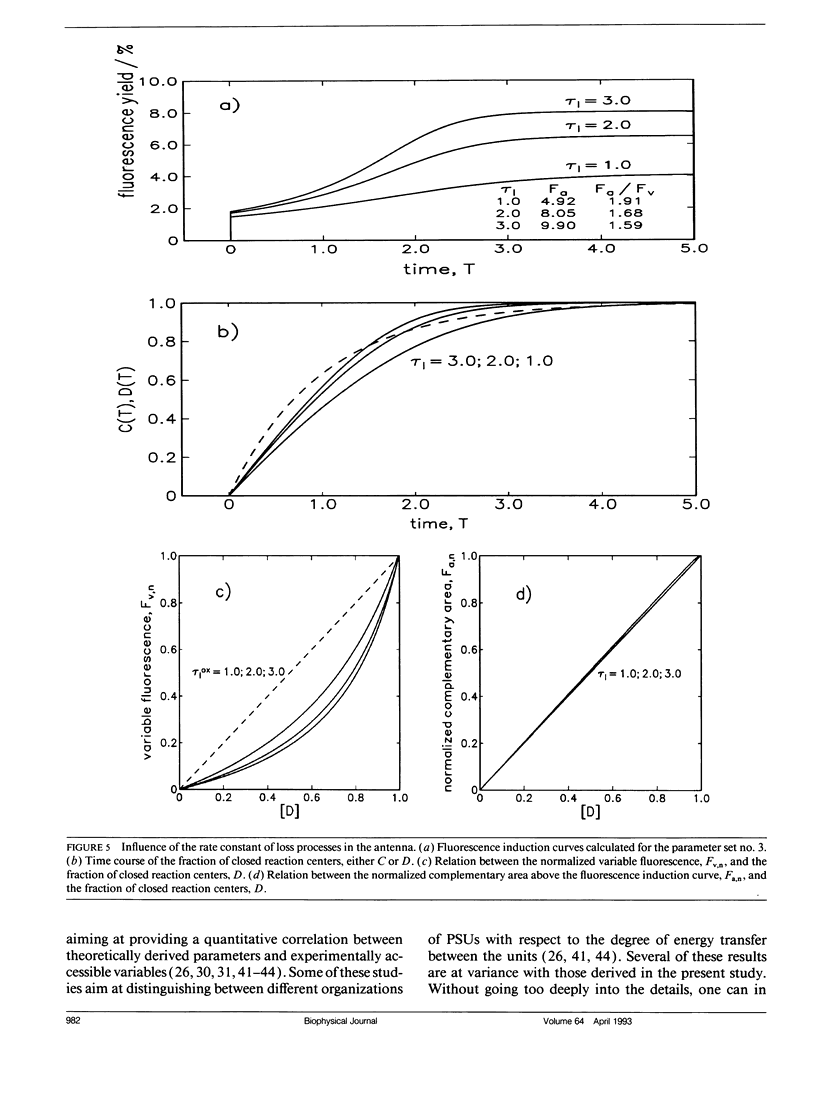
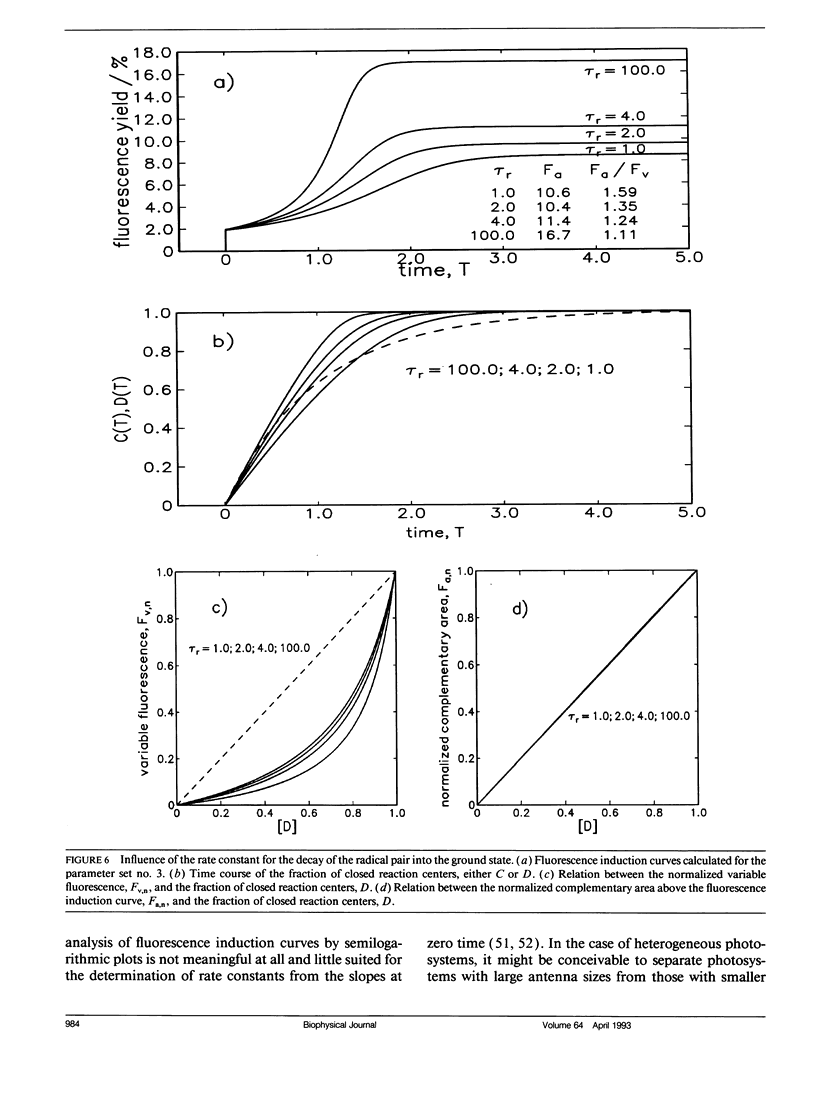
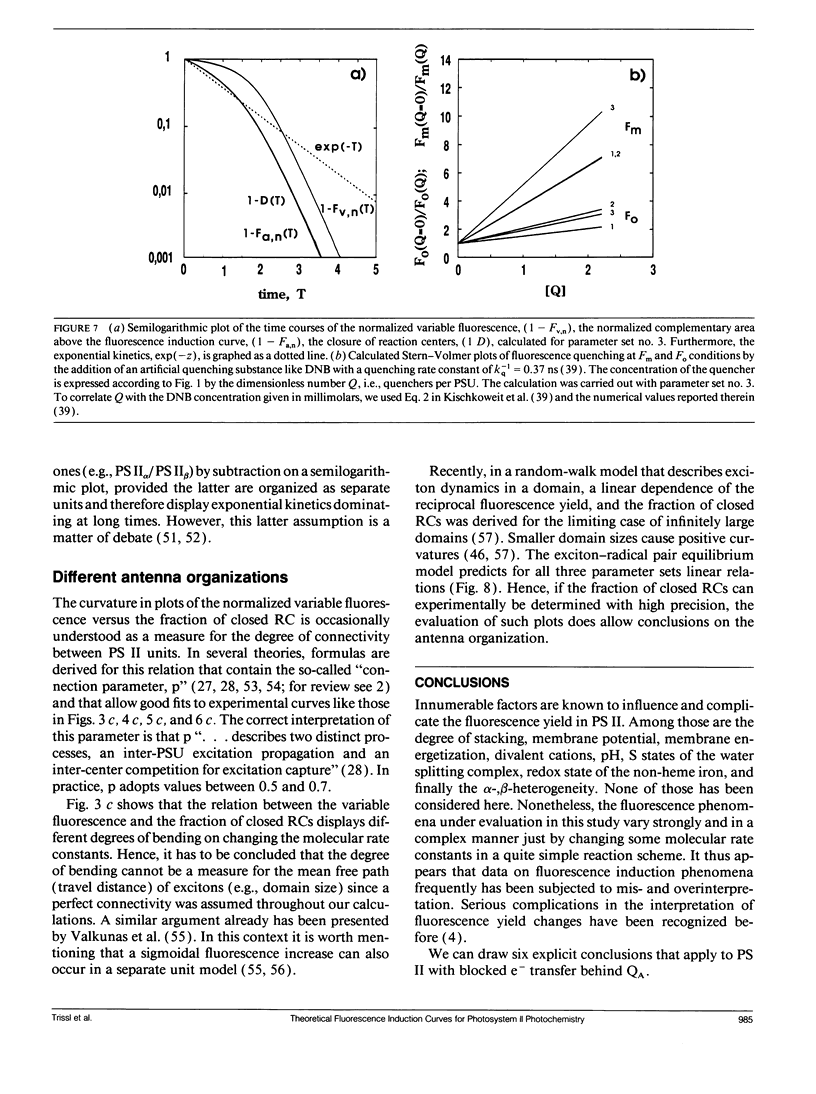
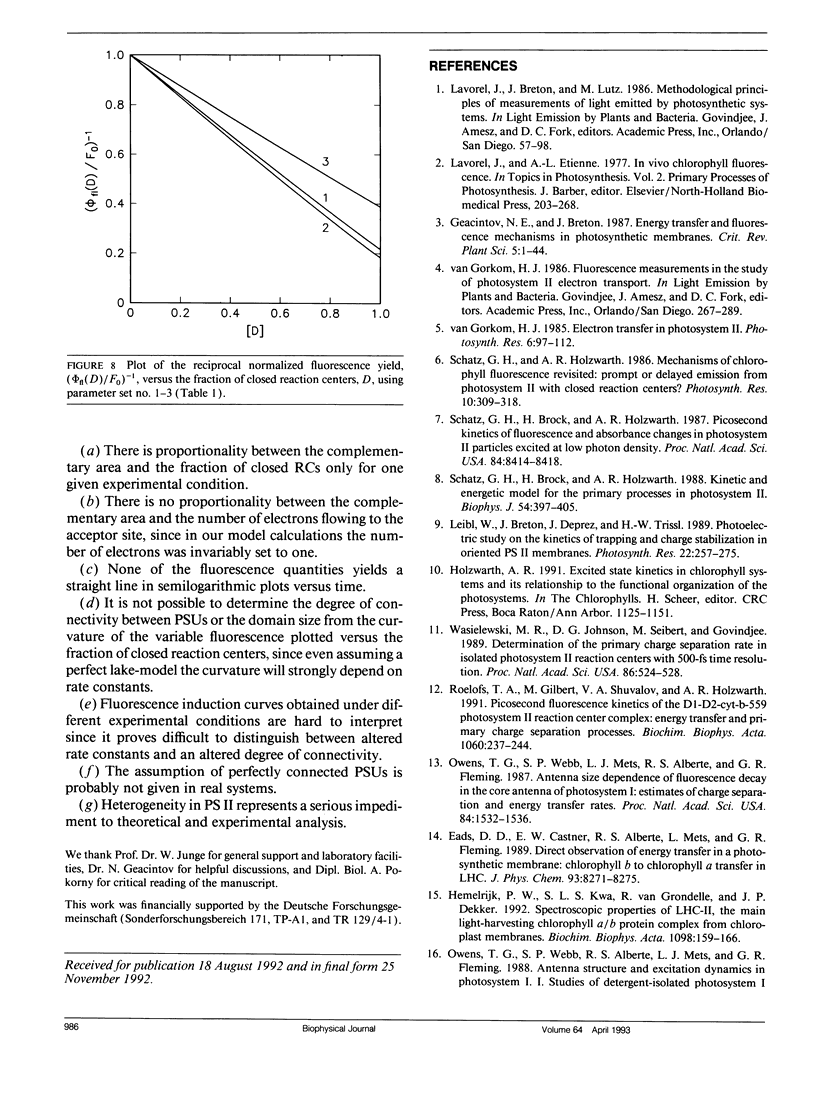
Fluorescence induction curves were calculated from a molecular model for the primary photophysical and photochemical processes of photosystem II that includes reversible exciton trapping by open (PHQA) and closed (PHQ-A) reaction centers (RCs), charge stabilization as well as quenching by oxidized (P+HQ(-)A) RCs. For the limiting case of perfectly connected photosynthetic units (“lake model”) and thermal equilibrium between the primary radical pair (P+H-) and the excited singlet state, the primary reactions can be mathematically formulated by a set of coupled ordinary differential equations (ODE). These were numerically solved for weak flashes in a recursive way to simulate experiments with continuous illumination. Using recently published values for the molecular rate constants, this procedure yielded the time dependence of closed RCs as well as of the fluorescence yield (= fluorescence induction curves). The theoretical curves displayed the same sigmoidal shapes as experimental fluorescence induction curves. From the time development of closed RCs and the fluorescence yield, it was possible to check currently assumed proportionalities between the fraction of closed RCs and either (a) the variable fluorescence, (b) the complementary area above the fluorescence induction curve, or (c) the complementary area normalized to the variable fluorescence. By changing selected molecular rate constants, it is shown that, in contrast to current beliefs, none of these correlations obeys simple laws. The time dependence of these quantities is strongly nonexponential. In the presence of substances that quench the excited state, the model predicts straight lines in Stern-Volmer plots. We further conclude that it is impossible to estimate the degree of physical interunit energy transfer from the sigmoidicity of the fluorescence induction curve or from the curvature of the variable fluorescence plotted versus the fraction of closed RCs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun P., Li Y. New results on the mode of action of 3,-(3,4-Dichlorophenyl)-1,1-dimethylurea in spinach chloroplasts. Biochim Biophys Acta. 1973 Jan 18;292(1):162–168. doi: 10.1016/0005-2728(73)90260-0. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Clayton R. K. An analysis of the relations between fluorescence and photochemistry during photosynthesis. J Theor Biol. 1967 Feb;14(2):173–186. doi: 10.1016/0022-5193(67)90112-9. [DOI] [PubMed] [Google Scholar]
- Hipkins M. F. Kinetic analysis of the chlorophyll fluorescence inductions from chloroplasts blocked with 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Biochim Biophys Acta. 1978 Jun 8;502(3):514–523. doi: 10.1016/0005-2728(78)90084-1. [DOI] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Jursinic P., Govindjee The rise in chlorophyll a fluorescence yield and decay in delayed light emission in tris-washed chloroplasts in the 6-100 microseconds time range after an excitation flash. Biochim Biophys Acta. 1977 Aug 10;461(2):253–267. doi: 10.1016/0005-2728(77)90175-x. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Lavorel J., Joliot P. A connected model of the photosynthetic unit. Biophys J. 1972 Jul;12(7):815–831. doi: 10.1016/S0006-3495(72)86125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Fork D. C. Photosynthetic units of sun and shade plants. Plant Physiol. 1981 Mar;67(3):580–583. doi: 10.1104/pp.67.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescene of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1966 May 12;120(1):23–33. doi: 10.1016/0926-6585(66)90273-1. [DOI] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Alberte R. S., Mets L., Fleming G. R. Antenna structure and excitation dynamics in photosystem I. I. Studies of detergent-isolated photosystem I preparations using time-resolved fluorescence analysis. Biophys J. 1988 May;53(5):733–745. doi: 10.1016/S0006-3495(88)83154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna size dependence of fluorescence decay in the core antenna of photosystem I: estimates of charge separation and energy transfer rates. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1532–1536. doi: 10.1073/pnas.84.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs T. A., Holzwarth A. R. In search of a putative long-lived relaxed radical pair state in closed photosystem II: Kinetic modeling of picosecond fluorescence data. Biophys J. 1990 Jun;57(6):1141–1153. doi: 10.1016/S0006-3495(90)82634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs T. A., Lee C. H., Holzwarth A. R. Global target analysis of picosecond chlorophyll fluorescence kinetics from pea chloroplasts: A new approach to the characterization of the primary processes in photosystem II alpha- and beta-units. Biophys J. 1992 May;61(5):1147–1163. doi: 10.1016/s0006-3495(92)81924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Kinetic and Energetic Model for the Primary Processes in Photosystem II. Biophys J. 1988 Sep;54(3):397–405. doi: 10.1016/S0006-3495(88)82973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld A., Rademaker H., Duysens L. N. Transfer and trapping of excitation energy in photosystem II as studied by chlorophyll alpha 2 fluorescence quenching by dinitrobenzene and carotenoid triplet. The matrix model. Biochim Biophys Acta. 1980 Dec 3;593(2):272–289. doi: 10.1016/0005-2728(80)90065-1. [DOI] [PubMed] [Google Scholar]
- Valkunas L., Geacintov N. E., France L., Breton J. The dependence of the shapes of fluorescence induction curves in chloroplasts on the duration of illumination pulses. Biophys J. 1991 Feb;59(2):397–408. doi: 10.1016/S0006-3495(91)82233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski M. R., Johnson D. G., Seibert M., Govindjee Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500-fs time resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(2):524–528. doi: 10.1073/pnas.86.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan G. A., Warden J. T., Duysens L. N. Kinetics of the fluorescence yield of chlorophyll a2 in spinach chloroplasts at liquid nitrogen temperature during and following a 16 micro second flash. Biochim Biophys Acta. 1973 Oct 19;325(1):120–125. doi: 10.1016/0005-2728(73)90157-6. [DOI] [PubMed] [Google Scholar]


