Abstract
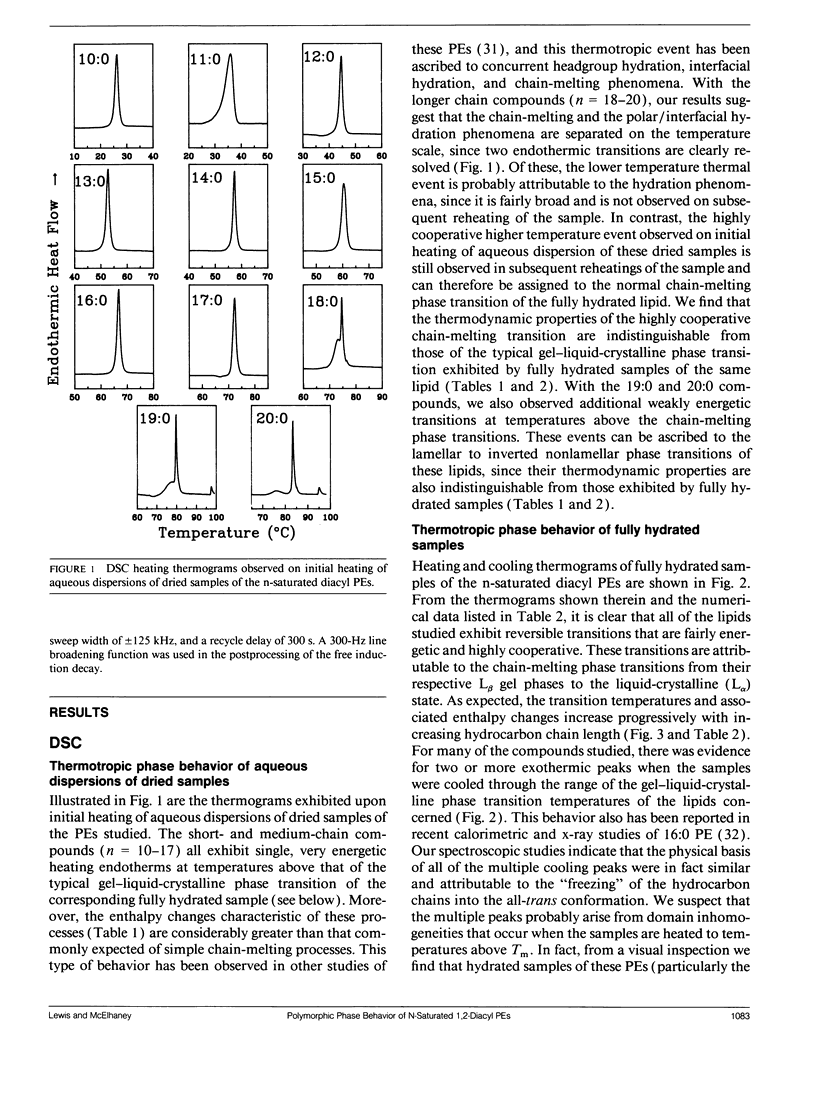
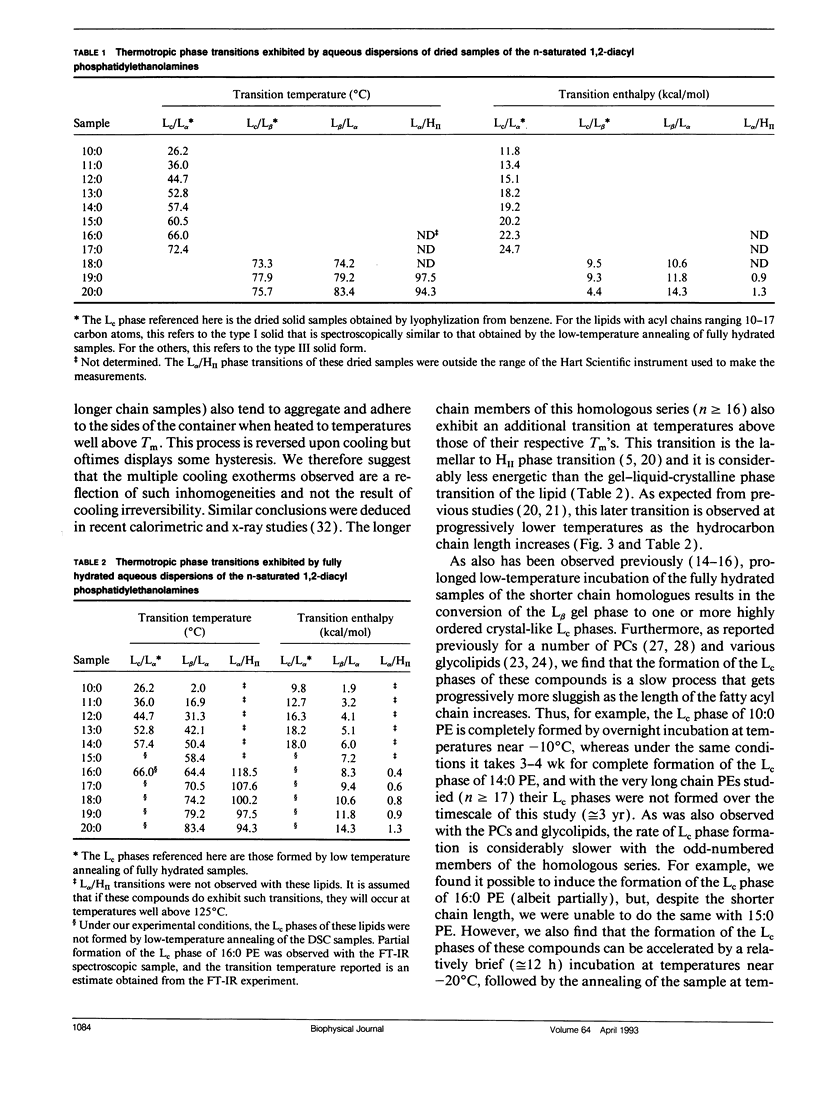
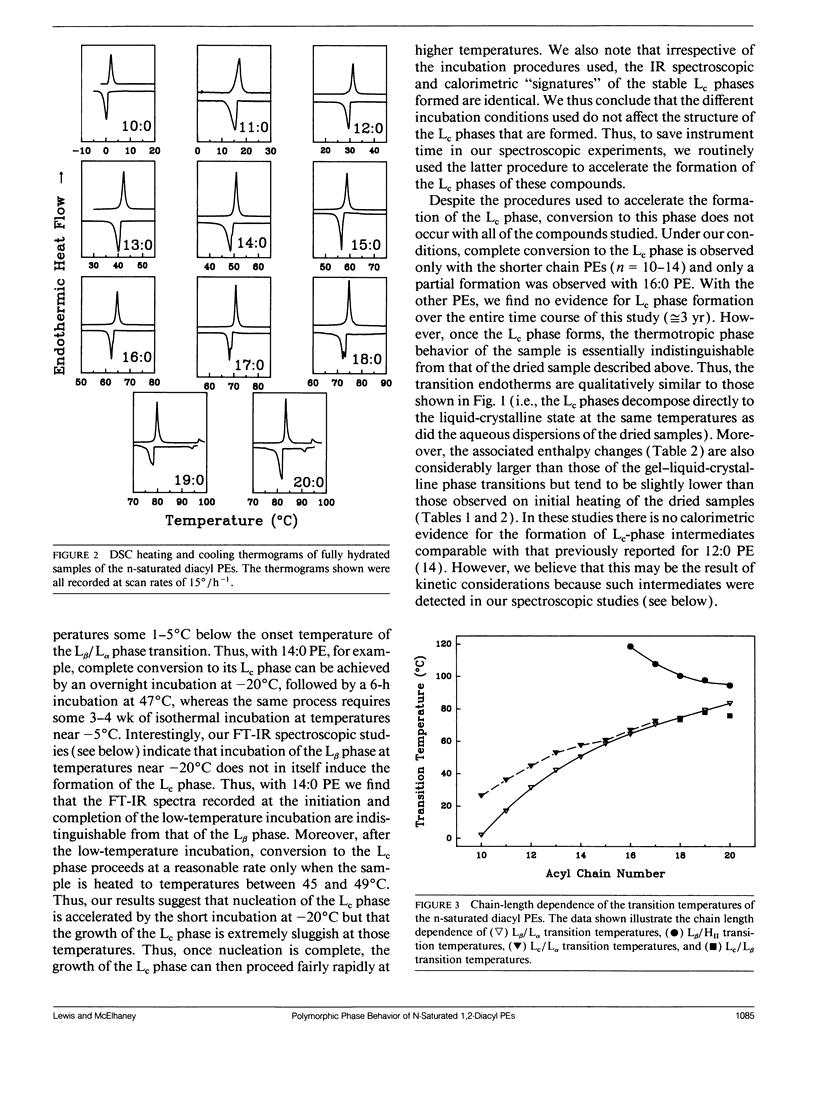
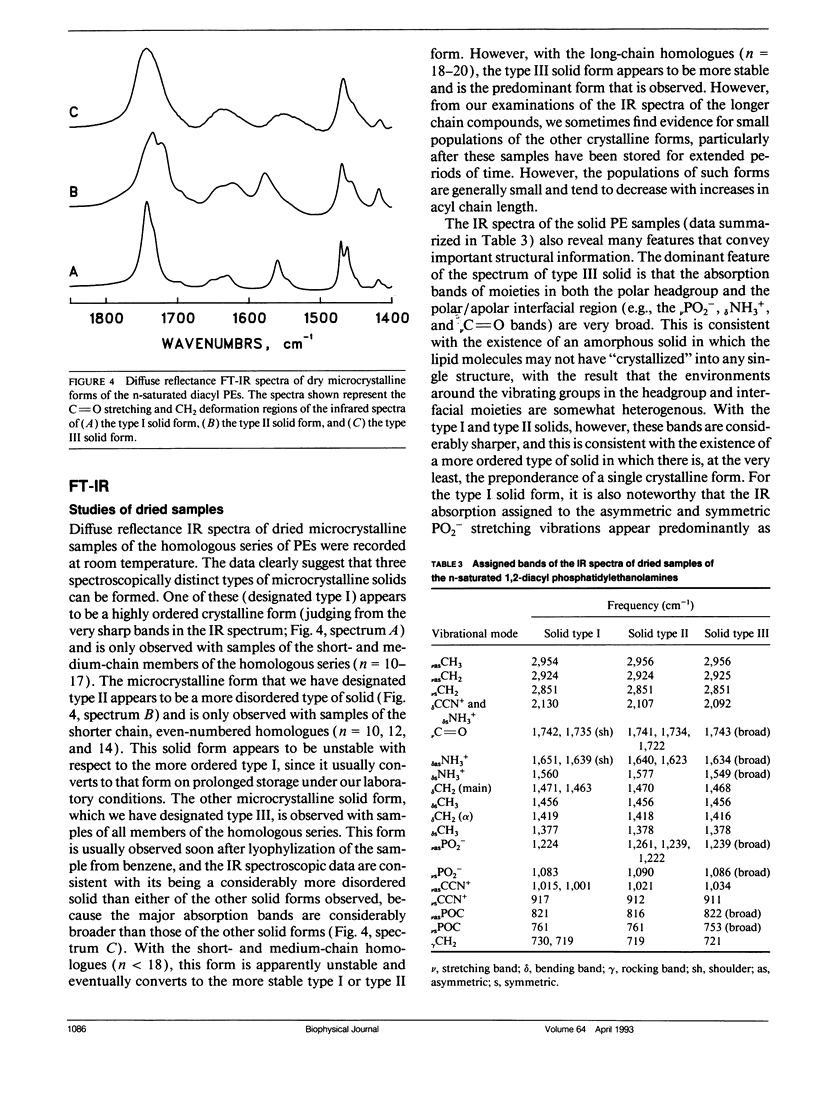
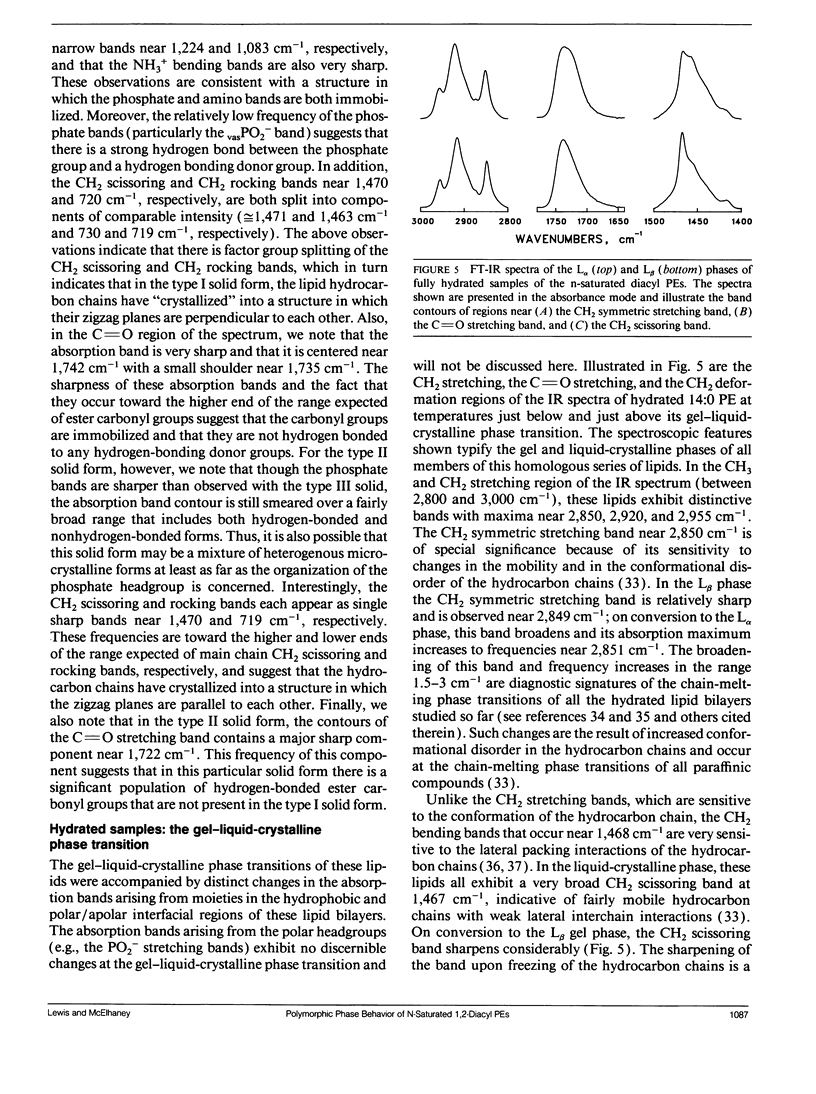
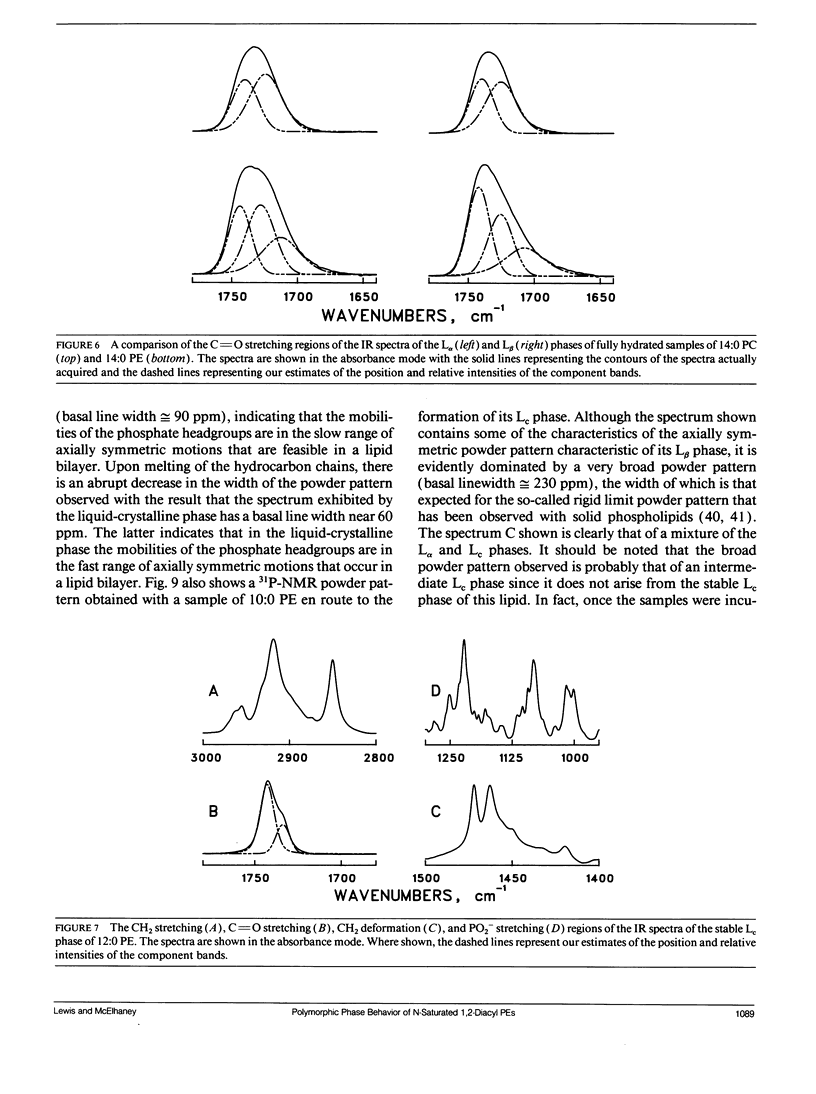
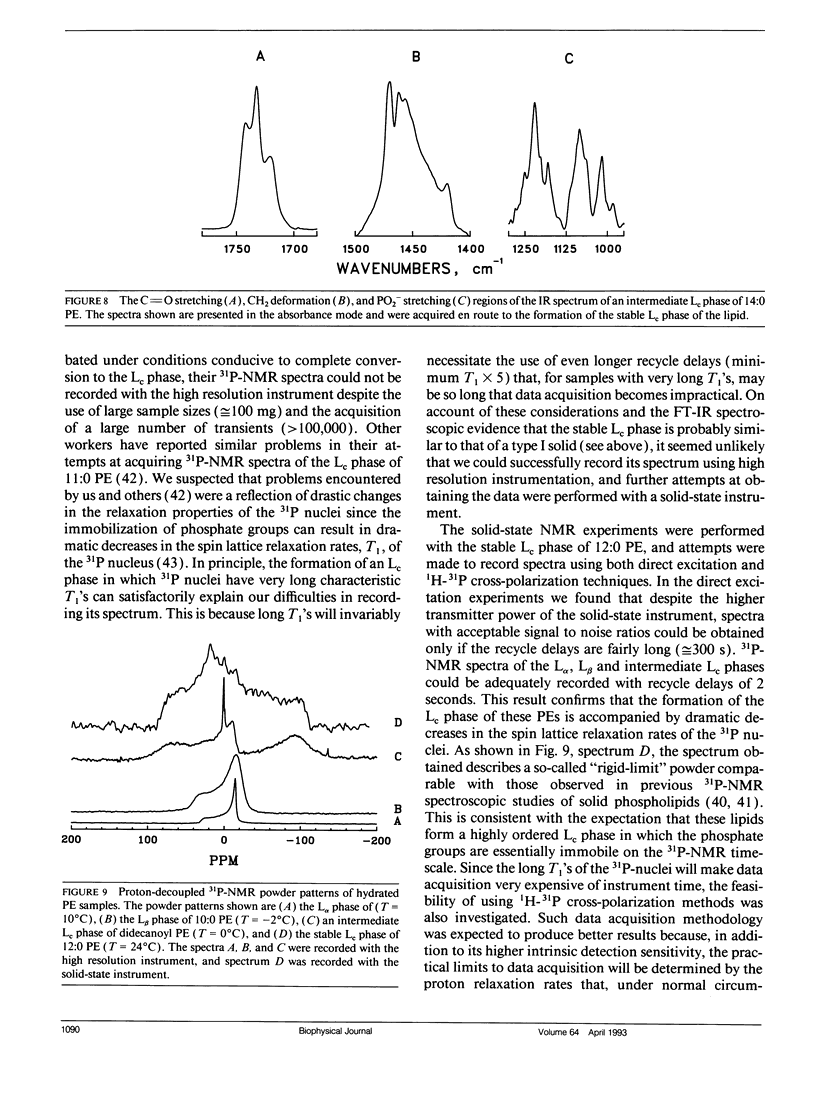
The polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines was investigated by differential scanning calorimetry, 31P-nuclear magnetic resonance, and Fourier transform infrared spectroscopy. Upon heating, aqueous dispersions of dried samples of the short- and medium-chain homologues (n < or = 17) exhibit single, highly energetic transitions from a dry, crystalline form to the fully hydrated, liquid-crystalline bilayer at temperatures higher than the lamellar gel-liquid-crystalline phase transition exhibited by fully hydrated samples. In contrast, the longer chain homologues (n > or = 18) first exhibit a transition from a dehydrated solid form to the hydrated L beta gel phase followed by the gel-liquid-crystalline phase transition normally observed with fully hydrated samples. The fully hydrated, aqueous dispersions of these lipids all exhibit reversible, fairly energetic gel-liquid-crystalline transitions at temperatures that are significantly higher than those of the corresponding phosphatidylcholines. In addition, at still higher temperatures, the longer chain members of this series (n > or = 16) exhibit weakly energetic transitions from the lamellar phase to an inverted nonlamellar phase. Upon appropriate incubation at low temperatures, aqueous dispersions of the shorter chain members of this homologous series (n < or = 16) form a highly ordered crystal-like phase that, upon heating, converts directly to the liquid-crystalline phase at the same temperature as do the aqueous dispersions of the dried lipid. The spectroscopic data indicate that unlike the n-saturated diacyl phosphatidylcholines, the stable crystal-like phases of this series of phosphatidylethanolamines describe an isostructural series in which the hydrocarbon chains are packed in an orthorhombic subcell and the headgroup and polar/apolar interfacial regions of the bilayer are effectively immobilized and substantially dehydrated. Our results suggest that many of the differences between the properties of these phosphatidylethanolamine bilayers and their phosphatidylcholine counterparts can be rationalized on the basis of stronger intermolecular interactions in the headgroup and interfacial regions of the phosphatidylethanolamine bilayers. These are probably the result of differences in the hydration and hydrogen bonding interactions involving the phosphorylethanolamine headgroup and moieties in the polar/apolar interfacial regions of phosphatidylethanolamine bilayers.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Effect of lipid structural modifications on their intermolecular hydrogen bonding interactions and membrane functions. Biochem Cell Biol. 1986 Jan;64(1):50–57. doi: 10.1139/o86-008. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can J Biochem. 1980 Oct;58(10):755–770. doi: 10.1139/o80-107. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Chang H., Epand R. M. The existence of a highly ordered phase in fully hydrated dilauroylphosphatidylethanolamine. Biochim Biophys Acta. 1983 Mar 9;728(3):319–324. doi: 10.1016/0005-2736(83)90501-1. [DOI] [PubMed] [Google Scholar]
- Chowdhry B. Z., Lipka G., Dalziel A. W., Sturtevant J. M. Multicomponent phase transitions of diacylphosphatidylethanolamine dispersions. Biophys J. 1984 May;45(5):901–904. doi: 10.1016/S0006-3495(84)84236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church S. E., Griffiths D. J., Lewis R. N., McElhaney R. N., Wickman H. H. X-ray structure study of thermotropic phases in isoacylphosphatidylcholine multibilayers. Biophys J. 1986 Mar;49(3):597–605. doi: 10.1016/S0006-3495(86)83687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. 31P nuclear magnetic resonance chemical shielding tensors of phosphorylethanolamine, lecithin, and related compounds: Applications to head-group motion in model membranes. Biochemistry. 1976 Mar 9;15(5):967–974. doi: 10.1021/bi00650a004. [DOI] [PubMed] [Google Scholar]
- Koynova R., Hinz H. J. Metastable behaviour of saturated phosphatidylethanolamines: a densitometric study. Chem Phys Lipids. 1990 Apr;54(1):67–72. doi: 10.1016/0009-3084(90)90061-u. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mannock D. A., McElhaney R. N., Turner D. C., Gruner S. M. Effect of fatty acyl chain length and structure on the lamellar gel to liquid-crystalline and lamellar to reversed hexagonal phase transitions of aqueous phosphatidylethanolamine dispersions. Biochemistry. 1989 Jan 24;28(2):541–548. doi: 10.1021/bi00428a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mantsch H. H., McElhaney R. N. Thermotropic phase behavior of phosphatidylcholines with omega-tertiary-butyl fatty acyl chains. Biophys J. 1989 Jul;56(1):183–193. doi: 10.1016/S0006-3495(89)82663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Structures of the subgel phases of n-saturated diacyl phosphatidylcholine bilayers: FTIR spectroscopic studies of 13C = O and 2H labeled lipids. Biophys J. 1992 Jan;61(1):63–77. doi: 10.1016/S0006-3495(92)81816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Subgel phases of n-saturated diacylphosphatidylcholines: a Fourier-transform infrared spectroscopic study. Biochemistry. 1990 Aug 28;29(34):7946–7953. doi: 10.1021/bi00486a024. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing omega-cyclohexyl fatty acids. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1985 Aug 27;24(18):4903–4911. doi: 10.1021/bi00339a027. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing cis-monounsaturated acyl chain homologues of oleic acid: differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1988 Feb 9;27(3):880–887. doi: 10.1021/bi00403a007. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 1. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7790–7799. doi: 10.1021/bi00486a003. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., Sen A., McElhaney R. N. The physical properties of glycosyldiacylglycerols. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(beta-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1988 Sep 6;27(18):6852–6859. doi: 10.1021/bi00418a030. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., McElhaney R. N. Differential scanning calorimetry and X-ray diffraction studies of a series of synthetic beta-D-galactosyl diacylglycerols. Biochem Cell Biol. 1991 Dec;69(12):863–867. doi: 10.1139/o91-128. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Hsi S. C., Butler K. W., Cameron D. G. Studies on the thermotropic behavior of aqueous phosphatidylethanolamines. Biochim Biophys Acta. 1983 Mar 9;728(3):325–330. doi: 10.1016/0005-2736(83)90502-3. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. An infrared spectroscopic study of the thermotropic phase behavior of phosphatidylcholines containing omega-cyclohexyl fatty acyl chains. Biochim Biophys Acta. 1989 Mar 27;980(1):42–49. doi: 10.1016/0005-2736(89)90198-3. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing dl-methyl anteisobranched fatty acids. 2. An infrared spectroscopy study. Biochemistry. 1987 Jun 30;26(13):4045–4049. doi: 10.1021/bi00387a045. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 2. Infrared and 31P NMR spectroscopic studies. Biochemistry. 1985 May 7;24(10):2440–2446. doi: 10.1021/bi00331a008. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., McElhaney R. N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem Phys Lipids. 1991 Mar;57(2-3):213–226. doi: 10.1016/0009-3084(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Marsh D. Analysis of the chainlength dependence of lipid phase transition temperatures: main and pretransitions of phosphatidylcholines; main and non-lamellar transitions of phosphatidylethanolamines. Biochim Biophys Acta. 1991 Feb 11;1062(1):1–6. doi: 10.1016/0005-2736(91)90326-4. [DOI] [PubMed] [Google Scholar]
- Marsh D., Seddon J. M. Gel-to-inverted hexagonal (L beta-HII) phase transitions in phosphatidylethanolamines and fatty acid-phosphatidylcholine mixtures, demonstrated by 31P-NMR spectroscopy and x-ray diffraction. Biochim Biophys Acta. 1982 Aug 25;690(1):117–123. doi: 10.1016/0005-2736(82)90245-0. [DOI] [PubMed] [Google Scholar]
- Mushayakarara E. C., Wong P. T., Mantsch H. H. Detection by high pressure infrared spectrometry of hydrogen-bonding between water and triacetyl glycerol. Biochem Biophys Res Commun. 1986 Jan 14;134(1):140–145. doi: 10.1016/0006-291x(86)90538-3. [DOI] [PubMed] [Google Scholar]
- Mushayakarara E., Albon N., Levin I. W. Effect of water on the molecular structure of a phosphatidylcholine hydrate. Raman spectroscopic analysis of the phosphate, carbonyl and carbon-hydrogen stretching mode regions of 1,2-dipalmitoylphosphatidylcholine dihydrate. Biochim Biophys Acta. 1982 Apr 7;686(2):153–159. doi: 10.1016/0005-2736(82)90107-9. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Theory of lipid monolayer and bilayer phase transitions: effect of headgroup interactions. J Membr Biol. 1976;27(3):233–250. doi: 10.1007/BF01869138. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Harlos K., Marsh D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J Biol Chem. 1983 Mar 25;258(6):3850–3854. [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Shyamsunder E., Gruner S. M., Tate M. W., Turner D. C., So P. T., Tilcock C. P. Observation of inverted cubic phase in hydrated dioleoylphosphatidylethanolamine membranes. Biochemistry. 1988 Apr 5;27(7):2332–2336. doi: 10.1021/bi00407a014. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Brown P. M., O'Leary T. J. Role of head group structure in the phase behavior of amino phospholipids. 1. Hydrated and dehydrated lamellar phases of saturated phosphatidylethanolamine analogues. Biochemistry. 1986 Jul 29;25(15):4249–4258. doi: 10.1021/bi00363a012. [DOI] [PubMed] [Google Scholar]
- Singer M. A., Finegold L., Rochon P., Racey T. J. The formation of multilamellar vesicles from saturated phosphatidylcholines and phosphatidylethanolamines: morphology and quasi-elastic light scattering measurements. Chem Phys Lipids. 1990 May;54(2):131–146. doi: 10.1016/0009-3084(90)90067-2. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Eikenberry E. F., Turner D. C., Shyamsunder E., Gruner S. M. Nonbilayer phases of membrane lipids. Chem Phys Lipids. 1991 Mar;57(2-3):147–164. doi: 10.1016/0009-3084(91)90073-k. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Nagle J. F. Metastability in the phase behavior of dimyristoylphosphatidylethanolamine bilayers. Biochemistry. 1984 Mar 27;23(7):1538–1541. doi: 10.1021/bi00302a030. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. 31P NMR relaxation studies of the activation of the coenzyme phosphate of glycogen phosphorylase. The role of motion of the bound phosphate. Biophys J. 1985 Dec;48(6):1019–1026. doi: 10.1016/S0006-3495(85)83864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Stephenson F. A., Lin H. N., Huang C. H. Phase metastability and supercooled metastable state of diundecanoylphosphatidylethanolamine bilayers. Biochim Biophys Acta. 1988 Aug 4;943(1):63–75. doi: 10.1016/0005-2736(88)90347-1. [DOI] [PubMed] [Google Scholar]
- Yao H., Hatta I., Koynova R., Tenchov B. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: II. On the fine structure of the phase transitions in hydrated dipalmitoylphosphatidylethanolamine. Biophys J. 1992 Mar;61(3):683–693. doi: 10.1016/S0006-3495(92)81873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


