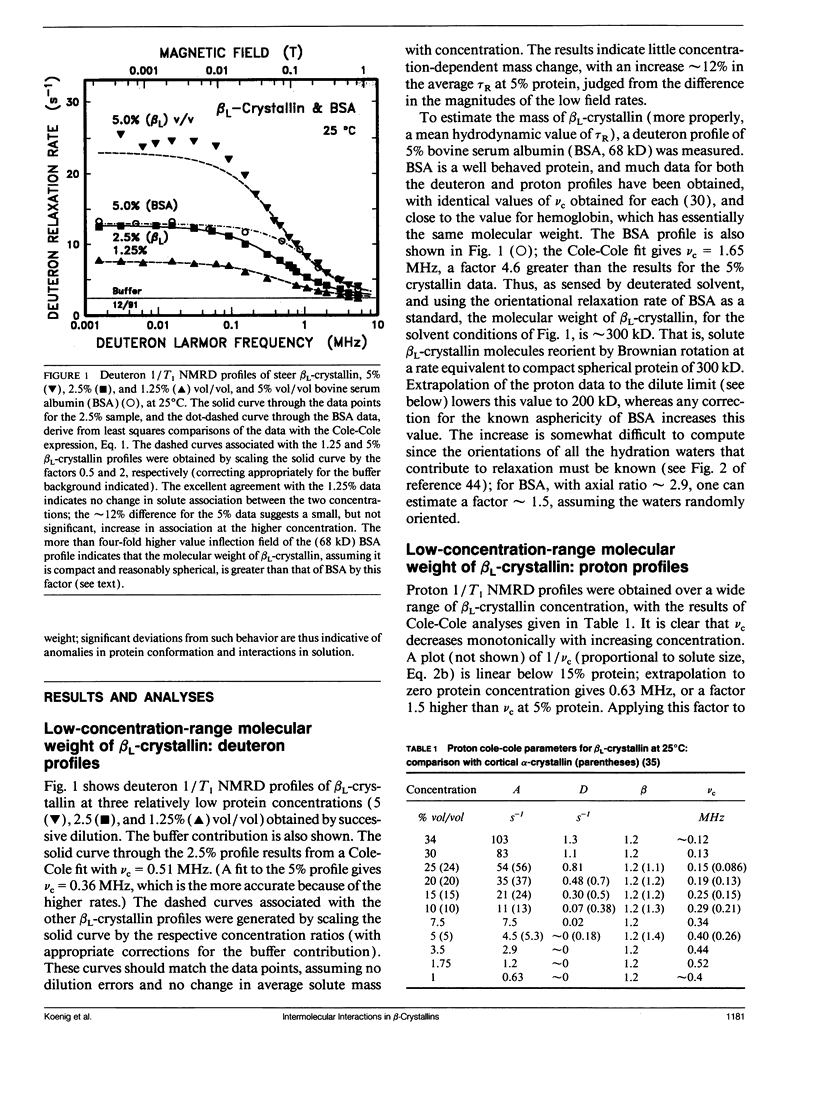
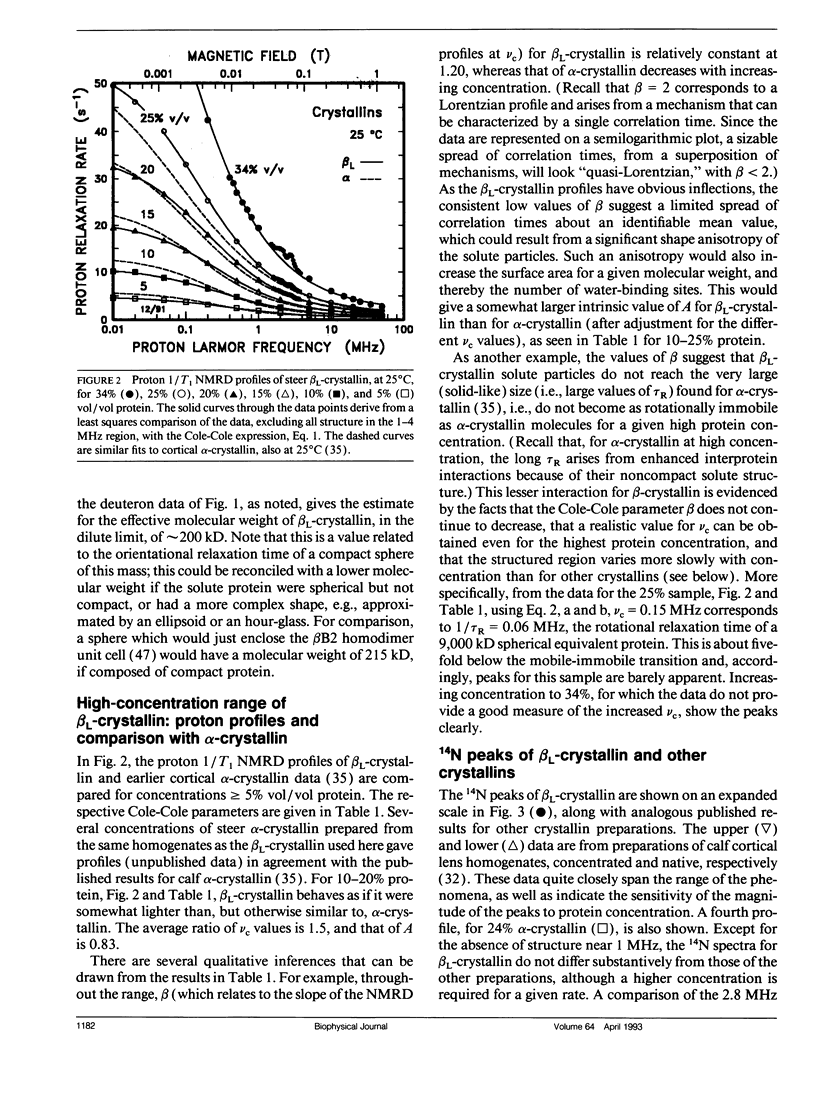
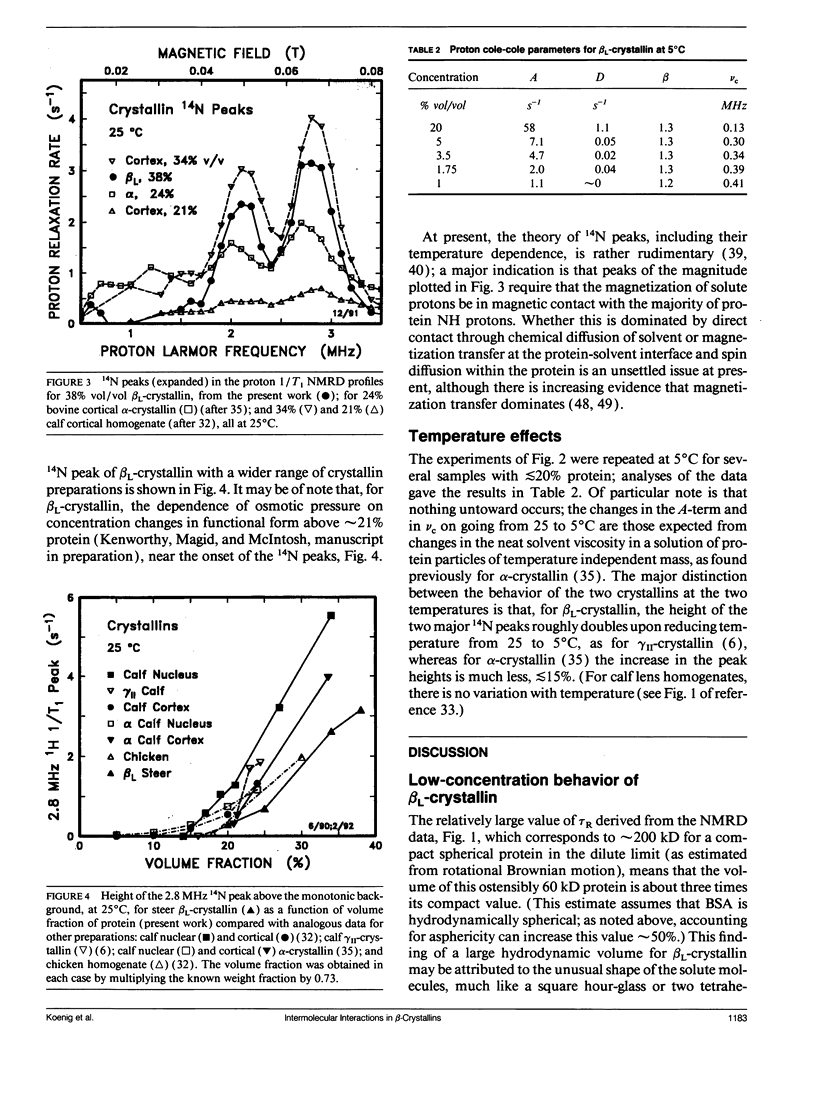
Abstract
We report the magnetic field dependence of 1/T1 of solvent water protons and deuterons (nuclear magnetic relaxation dispersion, or NMRD, profiles) for solutions of steer lens beta L-crystallin. Such data allow the study of intermolecular protein interactions over a wide concentration range, here 1-34% vol/vol, by providing a measure of the rotational relaxation time of solute macromolecules. We conclude that, for approximately less than 5% protein, the solute particles are noncompact, with a rotationally averaged volume approximately three times that of a compact 60-kD sphere. (Earlier results for alpha-crystallin, approximately 1,000 kD, from optical and osmotic measurements (Vérétout and Tardieu, 1989. J. Mol. Biol. 205:713-728), show a similar, approximately twofold, effect). At intermediate concentrations, to approximately 20% protein, there is evidence for limited association or oligomerization, as found for the structurally related gamma II-crystallin (Koenig et al. 1990. Biophys. J. 57:461-469), to a limiting size about two-thirds that of alpha-crystallin. The difference in NMRD behavior of the three classes of crystallins is consonant with their differing osmotic properties (Vérétout and Tardieu. J. Mol. Biol. 1989, 205:713-728; Kenworthy, McIntosh, and Magid. Biophys. J. 1992. 61:A477; Tardieu et al. 1992. Eur. Biophys. J. 21:1-12). We indicate how the unusual structures and interactions of these three classes of proteins can be combined to optimize transparency and minimize colloid osmotic difficulties in eye lens.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselbergs F. A., Koopmans M., van Venrooij W. J., Bloemendal H. Improved resolution of calf lens beta-crystallins. Exp Eye Res. 1979 Feb;28(2):223–228. doi: 10.1016/0014-4835(79)90133-7. [DOI] [PubMed] [Google Scholar]
- Bax B., Lapatto R., Nalini V., Driessen H., Lindley P. F., Mahadevan D., Blundell T. L., Slingsby C. X-ray analysis of beta B2-crystallin and evolution of oligomeric lens proteins. Nature. 1990 Oct 25;347(6295):776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. F., Clark J. I., Brown R. D., 3rd, Spiller M., Koenig S. H. Relaxometry of calf lens homogenates, including cross-relaxation by crystallin NH groups. Magn Reson Med. 1988 Sep;8(1):45–57. doi: 10.1002/mrm.1910080106. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Boerman O. C., Bloemendal H., de Jong W. W. Primary gene products of bovine beta-crystallin and reassociation behavior of its aggregates. Eur J Biochem. 1982 Nov 15;128(2-3):495–502. doi: 10.1111/j.1432-1033.1982.tb06992.x. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Hendriks I., Body P. Beta H- and beta L-crystallins are populations of aggregates. Exp Eye Res. 1990 Jun;50(6):711–713. doi: 10.1016/0014-4835(90)90118-e. [DOI] [PubMed] [Google Scholar]
- Brown R. D., 3rd, Koenig S. H. 1/T1 rho and low-field 1/T1 of tissue water protons arise from magnetization transfer to macromolecular solid-state broadened lines. Magn Reson Med. 1992 Nov;28(1):145–152. doi: 10.1002/mrm.1910280115. [DOI] [PubMed] [Google Scholar]
- Chiou S. H., Azari P., Himmel M. E., Lin H. K., Chang W. P. Physicochemical characterization of beta-crystallins from bovine lenses: hydrodynamic and aggregation properties. J Protein Chem. 1989 Feb;8(1):19–32. doi: 10.1007/BF01025076. [DOI] [PubMed] [Google Scholar]
- Croft L. R. The amino acid sequence of -crystallin (fraction II) from calf lens. Biochem J. 1972 Jul;128(4):961–970. doi: 10.1042/bj1280961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen H. P., Bax B., Slingsby C., Lindley P. F., Mahadevan D., Moss D. S., Tickle I. J. Structure of oligomeric beta B2-crystallin: an application of the T2 translation function to an asymmetric unit containing two dimers. Acta Crystallogr B. 1991 Dec 1;47(Pt 6):987–997. doi: 10.1107/s0108768191009163. [DOI] [PubMed] [Google Scholar]
- Hallenga K., Koenig S. H. Protein rotational relaxation as studied by solvent 1H and 2H magnetic relaxation. Biochemistry. 1976 Sep 21;15(19):4255–4264. doi: 10.1021/bi00664a019. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Beaulieu C. F., Brown R. D., 3rd, Spiller M. Oligomerization and conformation change in solutions of calf lens gamma II-crystallin. Results from 1/T1 nuclear magnetic relaxation dispersion profiles. Biophys J. 1990 Mar;57(3):461–469. doi: 10.1016/S0006-3495(90)82562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Adams D., Emerson D., Harrison C. G. Magnetic field dependence of 1/T1 of protons in tissue. Invest Radiol. 1984 Mar-Apr;19(2):76–81. doi: 10.1097/00004424-198403000-00002. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Gibson J. F., Ward R. J., Peters T. J. Relaxometry of ferritin solutions and the influence of the Fe3+ core ions. Magn Reson Med. 1986 Oct;3(5):755–767. doi: 10.1002/mrm.1910030511. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Spiller M., Chakrabarti B., Pande A. Intermolecular protein interactions in solutions of calf lens alpha-crystallin. Results from 1/T1 nuclear magnetic relaxation dispersion profiles. Biophys J. 1992 Mar;61(3):776–785. doi: 10.1016/S0006-3495(92)81882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Ugolini R. A unified view of relaxation in protein solutions and tissue, including hydration and magnetization transfer. Magn Reson Med. 1993 Jan;29(1):77–83. doi: 10.1002/mrm.1910290114. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd, Ugolini R. Magnetization transfer in cross-linked bovine serum albumin solutions at 200 MHz: a model for tissue. Magn Reson Med. 1993 Mar;29(3):311–316. doi: 10.1002/mrm.1910290306. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Bryant R. G., Hallenga K., Jacob G. S. Magnetic cross-relaxation among protons in protein solutions. Biochemistry. 1978 Oct 3;17(20):4348–4358. doi: 10.1021/bi00613a037. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. I. Apotransferrin. J Biol Chem. 1969 Jun 25;244(12):3283–3289. [PubMed] [Google Scholar]
- Koenig S. H. Theory of relaxation of mobile water protons induced by protein NH moieties, with application to rat heart muscle and calf lens homogenates. Biophys J. 1988 Jan;53(1):91–96. doi: 10.1016/S0006-3495(88)83069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R., Nalini V., Bax B., Driessen H., Lindley P. F., Blundell T. L., Slingsby C. High resolution structure of an oligomeric eye lens beta-crystallin. Loops, arches, linkers and interfaces in beta B2 dimer compared to a monomeric gamma-crystallin. J Mol Biol. 1991 Dec 20;222(4):1067–1083. doi: 10.1016/0022-2836(91)90594-v. [DOI] [PubMed] [Google Scholar]
- Morgan C. F., Schleich T., Caines G. H., Farnsworth P. N. Elucidation of intermediate (mobile) and slow (solidlike) protein motions in bovine lens homogenates by carbon-13 NMR spectroscopy. Biochemistry. 1989 Jun 13;28(12):5065–5074. doi: 10.1021/bi00438a025. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. The recruitment of crystallins: new functions precede gene duplication. Science. 1991 May 24;252(5009):1078–1079. doi: 10.1126/science.252.5009.1078. [DOI] [PubMed] [Google Scholar]
- Pierscionek B., Augusteyn R. C. Protein distribution patterns in concentric layers from single bovine lenses: changes with development and ageing. Curr Eye Res. 1988 Jan;7(1):11–23. doi: 10.3109/02713688809047015. [DOI] [PubMed] [Google Scholar]
- Prouty M. S., Schechter A. N., Parsegian V. A. Chemical potential measurements of deoxyhemoglobin S polymerization. Determination of the phase diagram of an assembling protein. J Mol Biol. 1985 Aug 5;184(3):517–528. doi: 10.1016/0022-2836(85)90298-0. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Bateman O. A. Quaternary interactions in eye lens beta-crystallins: basic and acidic subunits of beta-crystallins favor heterologous association. Biochemistry. 1990 Jul 17;29(28):6592–6599. doi: 10.1021/bi00480a007. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Simpson A., Ferszt A., Bateman O. A., Nalini V. Molecular interactions in the eye lens. Biochem Soc Trans. 1991 Nov;19(4):853–858. doi: 10.1042/bst0190853. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Vérétout F., Krop B., Slingsby C. Protein interactions in the calf eye lens: interactions between beta-crystallins are repulsive whereas in gamma-crystallins they are attractive. Eur Biophys J. 1992;21(1):1–12. doi: 10.1007/BF00195438. [DOI] [PubMed] [Google Scholar]
- Vérétout F., Delaye M., Tardieu A. Molecular basis of eye lens transparency. Osmotic pressure and X-ray analysis of alpha-crystallin solutions. J Mol Biol. 1989 Feb 20;205(4):713–728. doi: 10.1016/0022-2836(89)90316-1. [DOI] [PubMed] [Google Scholar]
- Vérétout F., Tardieu A. The protein concentration gradient within eye lens might originate from constant osmotic pressure coupled to differential interactive properties of crystallins. Eur Biophys J. 1989;17(2):61–68. doi: 10.1007/BF00257103. [DOI] [PubMed] [Google Scholar]
- Winter F., Kimmich R. NMR field-cycling relaxation spectroscopy of bovine serum albumin, muscle tissue, Micrococcus luteus and yeast. 14N1H-quadrupole dips. Biochim Biophys Acta. 1982 Nov 24;719(2):292–298. doi: 10.1016/0304-4165(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr Age-related changes in the polypeptide composition of beta-crystallin from bovine lens. Exp Eye Res. 1978 May;26(5):537–546. doi: 10.1016/0014-4835(78)90063-5. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Sidbury J. B., Jr Structure of calf lens beta-crystallins. Exp Eye Res. 1973 Jul;16(3):207–214. doi: 10.1016/0014-4835(73)90215-7. [DOI] [PubMed] [Google Scholar]


