Abstract
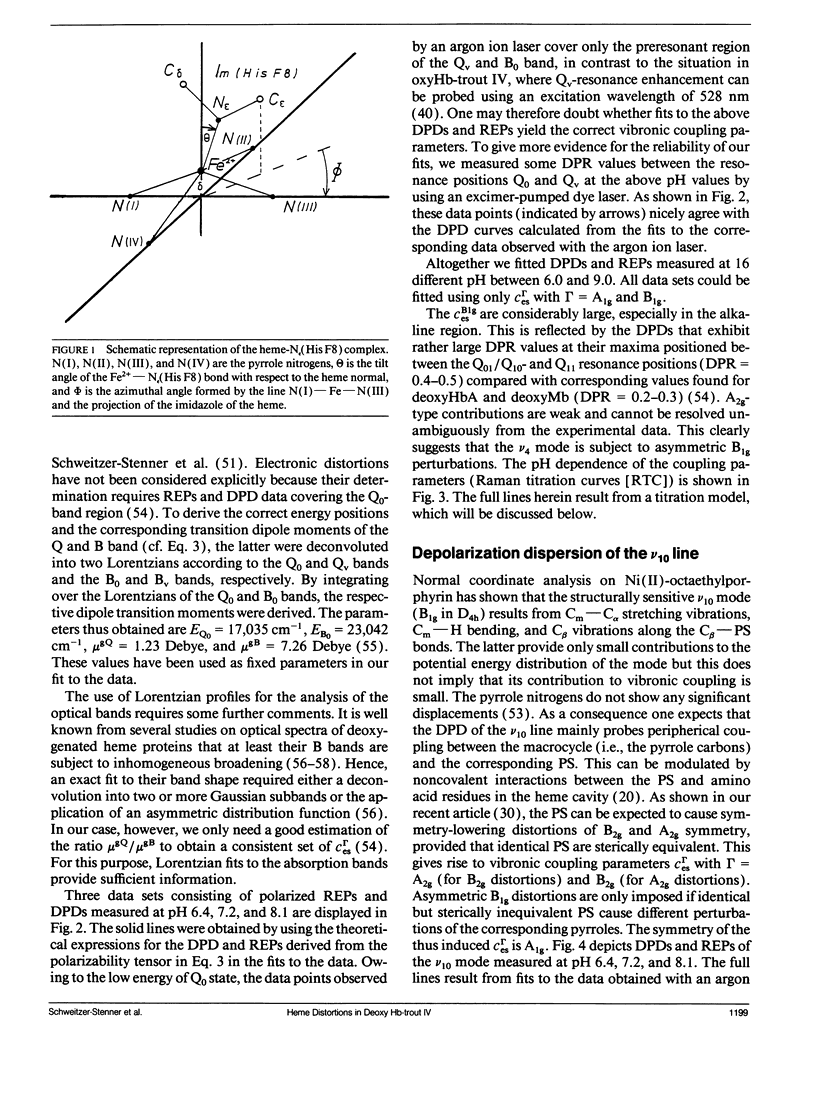
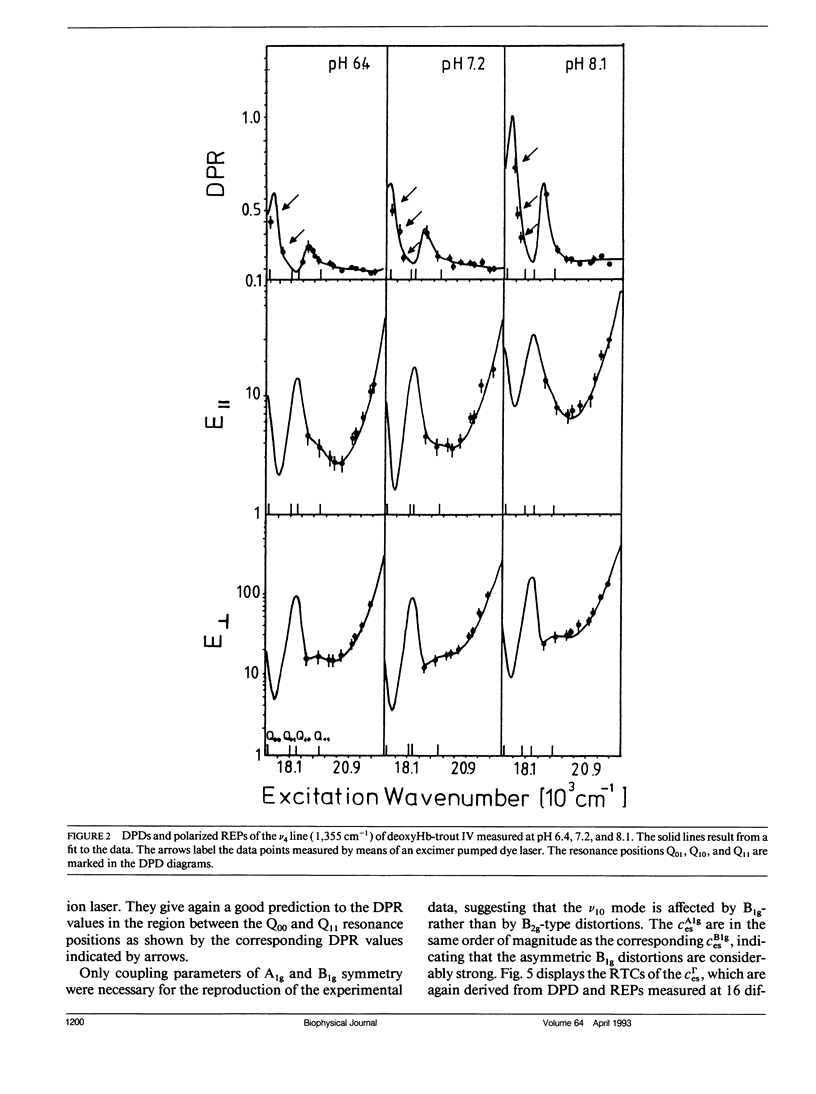
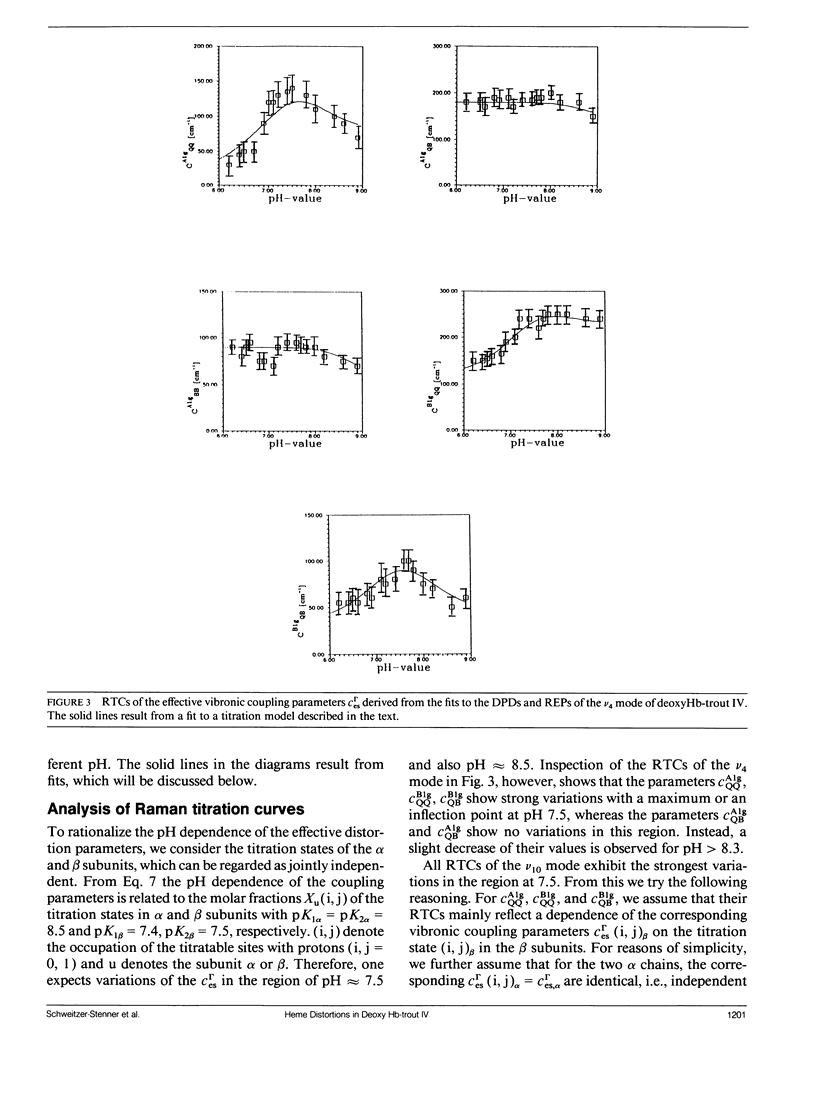
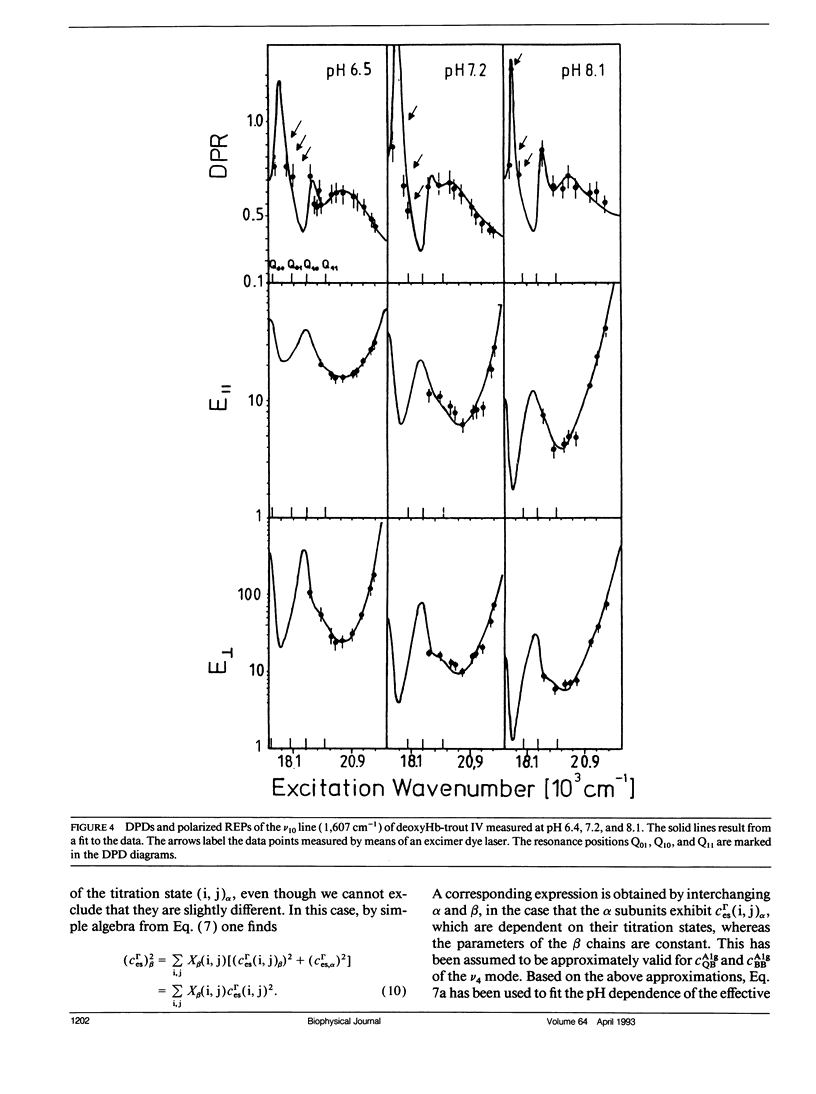
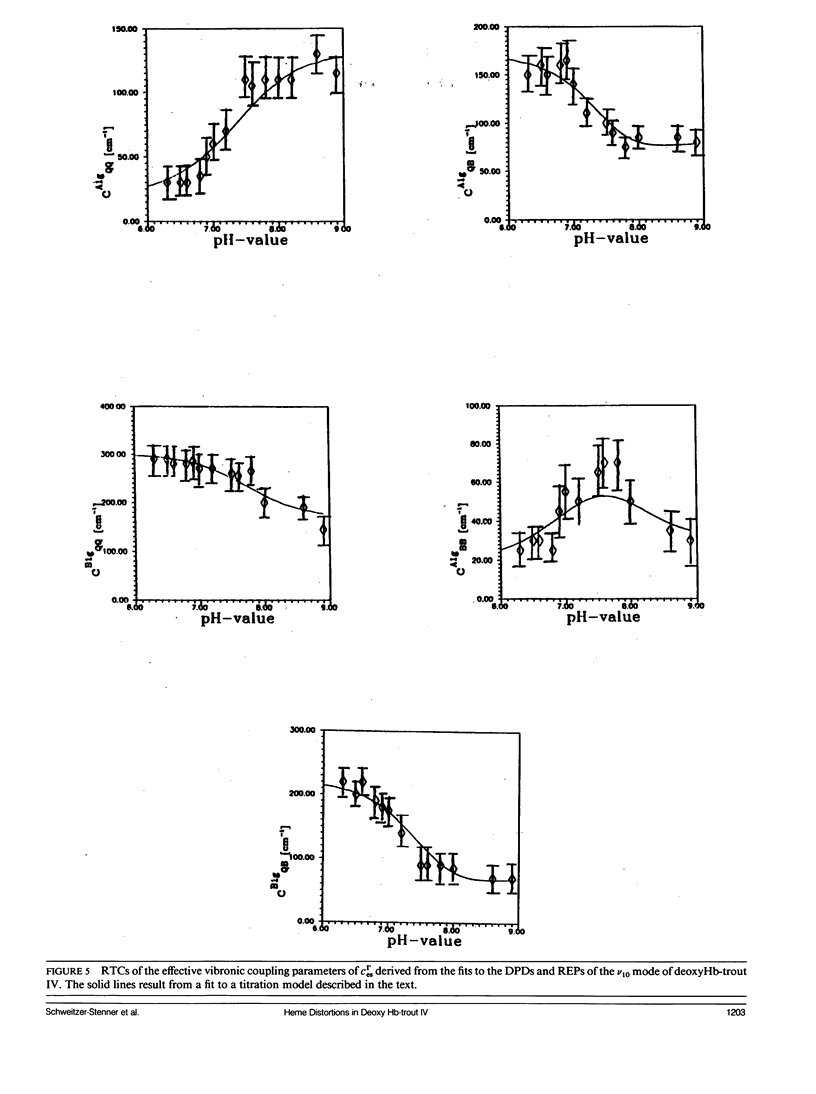
The depolarization ratios of heme protein Raman lines arising from vibrations of the heme group exhibit significant dependence on the excitation wavelength. From the analysis of this depolarization ratio dispersion, one obtains information about symmetry-lowering distortions δQΓ of the heme group that can be classified in terms of the symmetry races Γ = A1g, B1g, B2g, and A2g in D4h symmetry. The heme-protein interaction can be changed by the protonation of distinct amino acid side chains (i.e., for instance the Bohr groups in hemoglobin derivates), which gives rise to specific static heme distortions for each protonation state. From the Raman dispersion data, it is possible to obtain parameters by fitting to a theoretical expression of the Raman tensor, which provide information on these static distortions and also about the pK values of the involved titrable side chains. We have applied this method to the ν4 (1,355 cm-1) and ν10 (1,620 cm-1) lines of deoxygenated hemoglobin of the fourth component of trout and have measured their depolarization ratio dispersion as a function of pH between 6 and 9. From the pH dependence of the thus derived parameters, we obtain pK values identical to those of the Bohr groups, which were earlier derived from the corresponding O2-binding isotherms. These are pKα1 = pKα2 = 8.5 for the α and pKβ1 = 7.5, pKβ2 = 7.4 for the β chains. We also obtain the specific distortion parameters for each protonation state. As shown in earlier studies, the ν4 mode mainly probes distortions from interactions between the proximal histidine and atoms of the heme core (i.e., the nitrogens and the Cα atoms of the pyrroles). Group theoretical argumentation allows us to relate specific changes of the imidazole geometry as determined by its tilt and azimuthal angle and the iron-out-of-plane displacement to distinct variations of the normal distortions δQΓ derived from the Raman dispersion data. Thus, we found that the pH dependence of the heme distortions δQA1g (totally symmetric) and δQB1g (asymmetric) is caused by variations of the azimuthal rather than the tilt angle of the Fe-His (F8) bond. In contrast to this, the ν10 line mainly monitors changes resulting from the interaction between peripheral substituents of the porphyrin macrocycle (vinyl). From the pH dependence of the parameters, it is possible to separately identify distortions δQΓ affecting the hemes in the α and β chains, respectively. From this, we find that in the α subunit structural changes induced on protonation of the corresponding Bohr groups are mainly transferred via the Fe—Nε bond and give rise to changes in the azimuthal angle. In the β subunit, however, in addition, structural changes of the heme pocket arise, which most probably result from protonation of the imidazole of the COOH-terminal His (HC3 β). This rearranges the net of H bonds between His HC3 β, Ser (F9 β), and Glu (F7 β).
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Smith F. R. Resolving pathways of functional coupling within protein assemblies by site-specific structural perturbation. Biophys J. 1986 Jan;49(1):155–165. doi: 10.1016/S0006-3495(86)83631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli F., Falcioni G., Giardina B., Brunori M. Thermodynamic characterization of the allosteric transition in trout hemoglobin. Eur Biophys J. 1986;13(4):245–249. doi: 10.1007/BF00260371. [DOI] [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Bosenbeck M., Schweitzer-Stenner R., Dreybrodt W. pH-induced conformational changes of the Fe(2+)-N epsilon (His F8) linkage in deoxyhemoglobin trout IV detected by the Raman active Fe(2+)-N epsilon (His F8) stretching mode. Biophys J. 1992 Jan;61(1):31–41. doi: 10.1016/S0006-3495(92)81813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Coletta M., Giardina B., Wyman J. A macromolecular transducer as illustrated by trout hemoglobin IV. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4310–4312. doi: 10.1073/pnas.75.9.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M. Molecular adaptation to physiological requirements: the hemoglobin system of trout. Curr Top Cell Regul. 1975;9:1–39. doi: 10.1016/b978-0-12-152809-6.50008-1. [DOI] [PubMed] [Google Scholar]
- Chavez M. D., Courtney S. H., Chance M. R., Kiula D., Nocek J., Hoffman B. M., Friedman J. M., Ondrias M. R. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe-Mn hybrid hemoglobins. Biochemistry. 1990 May 22;29(20):4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys Chem. 1986 Aug;24(3):259–275. doi: 10.1016/0301-4622(86)85031-1. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Henry E. R., Hofrichter J. Application of linear free energy relations to protein conformational changes: the quaternary structural change of hemoglobin. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4472–4475. doi: 10.1073/pnas.88.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Campbell B. F., Noble R. W. A possible new control mechanism suggested by resonance Raman spectra from a deep ocean fish hemoglobin. Biophys Chem. 1990 Aug 31;37(1-3):43–59. doi: 10.1016/0301-4622(90)88006-e. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld J., Stanley H. E. A general approach to co-operativity and its application to the oxygen equilibrium of hemoglobin and its effectors. J Mol Biol. 1974 Jan 15;82(2):231–265. doi: 10.1016/0022-2836(74)90343-x. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Ackers G. K. Thermodynamic analysis of human hemoglobins in terms of the Perutz mechanism: extensions of the Szabo--Karplus model to include subunit assembly. Biochemistry. 1982 Jan 19;21(2):201–211. doi: 10.1021/bi00531a001. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Fogg J. H., Perutz M. F. Role of C-terminal histidine in the alkaline Bohr effect of human hemoglobin. Biochemistry. 1980 Jul 8;19(14):3189–3183. doi: 10.1021/bi00555a013. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski L. D., Noble R. W. The contribution of histidine (HC3) (146 beta) to the R state Bohr effect of human hemoglobin. J Biol Chem. 1982 Aug 10;257(15):8891–8895. [PubMed] [Google Scholar]
- Lee A. W., Karplus M., Poyart C., Bursaux E. Analysis of proton release in oxygen binding by hemoglobin: implications for the cooperative mechanism. Biochemistry. 1988 Feb 23;27(4):1285–1301. doi: 10.1021/bi00404a031. [DOI] [PubMed] [Google Scholar]
- Moffat J. K. Structure and functional properties of chemically modified horse hemoglobin. II. X-ray studies. J Mol Biol. 1971 May 28;58(1):79–88. doi: 10.1016/0022-2836(71)90233-6. [DOI] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Eaton W. A. Time-resolved optical spectroscopy and structural dynamics following photodissociation of carbonmonoxyhemoglobin. Biophys Chem. 1988 Feb;29(1-2):63–76. doi: 10.1016/0301-4622(88)87025-x. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Shelnutt J. A., Simon S. R. Quaternary-transformation-induced changes at the heme in deoxyhemoglobins. Biochemistry. 1982 Jul 6;21(14):3428–3437. doi: 10.1021/bi00257a028. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Brunori M. Stereochemistry of cooperative effects in fish an amphibian haemoglobins. Nature. 1982 Sep 30;299(5882):421–426. doi: 10.1038/299421a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Powers L., Chance B., Chance M., Campbell B., Friedman J., Khalid S., Kumar C., Naqui A., Reddy K. S., Zhou Y. Kinetic, structural, and spectroscopic identification of geminate states of myoglobin: a ligand binding site on the reaction pathway. Biochemistry. 1987 Jul 28;26(15):4785–4796. doi: 10.1021/bi00389a028. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R. Allosteric linkage-induced distortions of the prosthetic group in haem proteins as derived by the theoretical interpretation of the depolarization ratio in resonance Raman scattering. Q Rev Biophys. 1989 Nov;22(4):381–479. doi: 10.1017/s0033583500003164. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Dannemann U., Dreybrodt W. Investigation of heme distortions and heme-protein coupling in the isolated subunits of oxygenated human hemoglobin by resonance Raman dispersion spectroscopy. Biochemistry. 1992 Jan 28;31(3):694–702. doi: 10.1021/bi00118a009. [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Dreybrodt W. An extended Monod-Wyman-Changeaux-model expressed in terms of the Herzfeld-Stanley formalism applied to oxygen and carbonmonoxide binding curves of hemoglobin trout IV. Biophys J. 1989 Apr;55(4):691–701. doi: 10.1016/S0006-3495(89)82868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Wedekind D., Dreybrodt W. Correspondence of the pK values of oxyHb-titration states detected by resonance Raman scattering to kinetic data of ligand dissociation and association. Biophys J. 1986 May;49(5):1077–1088. doi: 10.1016/S0006-3495(86)83736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Wedekind D., Dreybrodt W. Detection of the heme perturbations caused by the quaternary R----T transition in oxyhemoglobin trout IV by resonance Raman scattering. Biophys J. 1989 Apr;55(4):703–712. doi: 10.1016/S0006-3495(89)82869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Wedekind D., Dreybrodt W. The influence of structural variations in the F- and FG-helix of the beta-subunit modified oxyHb-NES on the heme structure detected by resonance Raman spectroscopy. Eur Biophys J. 1989;17(2):87–100. doi: 10.1007/BF00257106. [DOI] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Shih T., Jones R. T., Bonaventura J., Bonaventura C., Schneider R. G. Involvement of His HC3 (146) beta in the Bohr effect of human hemoglobin. Studies of native and N-ethylmaleimide-treated hemoglobin A and hemoglobin Cowtown (beta 146 His replaced by Leu). J Biol Chem. 1984 Jan 25;259(2):967–974. [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Szabo A., Karplus M. Analysis of cooperativity in hemoglobin. Valency hybrids, oxidation, and methemoglobin replacement reactions. Biochemistry. 1975 Mar 11;14(5):931–940. doi: 10.1021/bi00676a009. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energy-structure correlation in metalloporphyrins and the control of oxygen binding by hemoglobin. Proc Natl Acad Sci U S A. 1977 May;74(5):1789–1793. doi: 10.1073/pnas.74.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind D., Schweitzer-Stenner R., Dreybrodt W. Heme-apoprotein interaction in the modified oxyhemoglobin-bis(N-maleimidomethyl)ether and in oxyhemoglobin at high Cl-concentration detected by resonance Raman scattering. Biochim Biophys Acta. 1985 Aug 23;830(3):224–232. doi: 10.1016/0167-4838(85)90278-x. [DOI] [PubMed] [Google Scholar]
- Wyman J., Gill S. J., Gaud H. T., Colosimo A., Giardina B., Kupier H. A., Brunori M. Thermodynamics of ligand binding and allosteric transition in hemoglobins. Reaction of Hb trout IV with CO. J Mol Biol. 1978 Sep 5;124(1):161–175. doi: 10.1016/0022-2836(78)90154-7. [DOI] [PubMed] [Google Scholar]
- Zhang N. Q., Ferrone F. A., Martino A. J. Allosteric kinetics and equilibria differ for carbon monoxide and oxygen binding to hemoglobin. Biophys J. 1990 Aug;58(2):333–340. doi: 10.1016/S0006-3495(90)82380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Naggar S., Dreybrodt W., Schweitzer-Stenner R. Haem-apoprotein interactions detected by resonance Raman scattering in Mb- and Hb-derivates lacking the saltbridge His146 beta-Asp94 beta. Eur Biophys J. 1985;12(1):43–49. doi: 10.1007/BF00254094. [DOI] [PubMed] [Google Scholar]


