Abstract
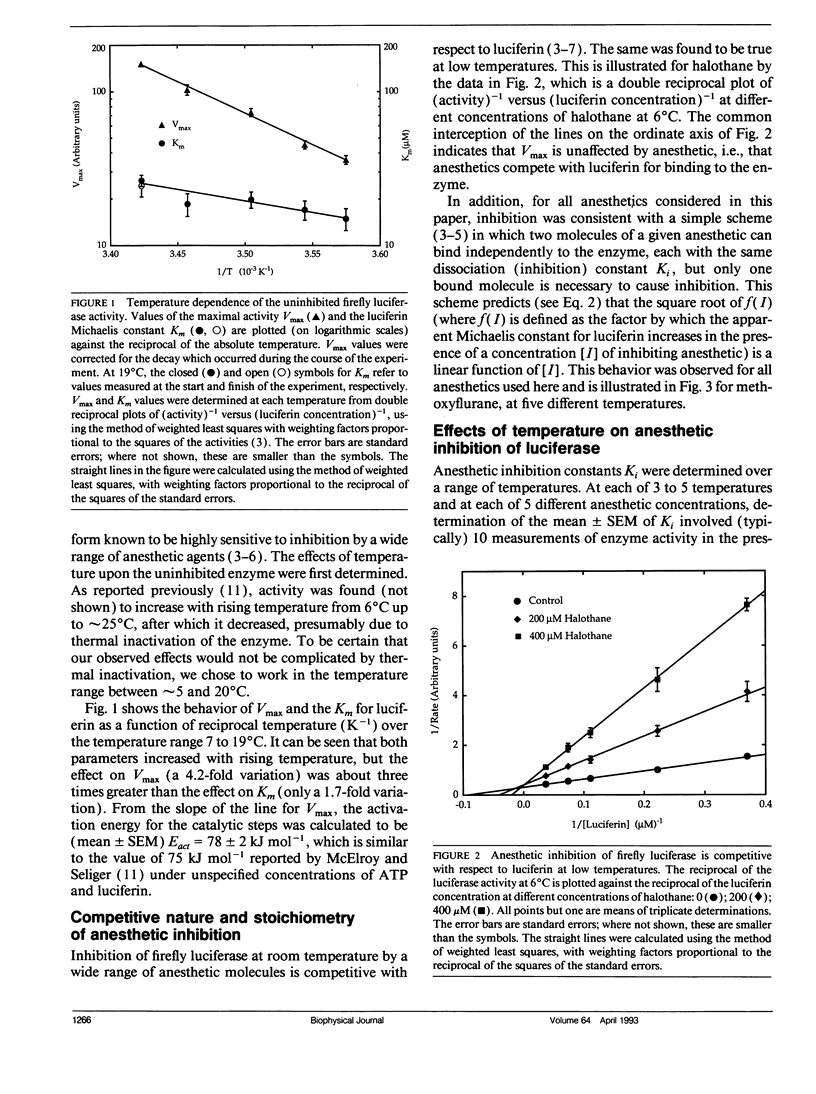
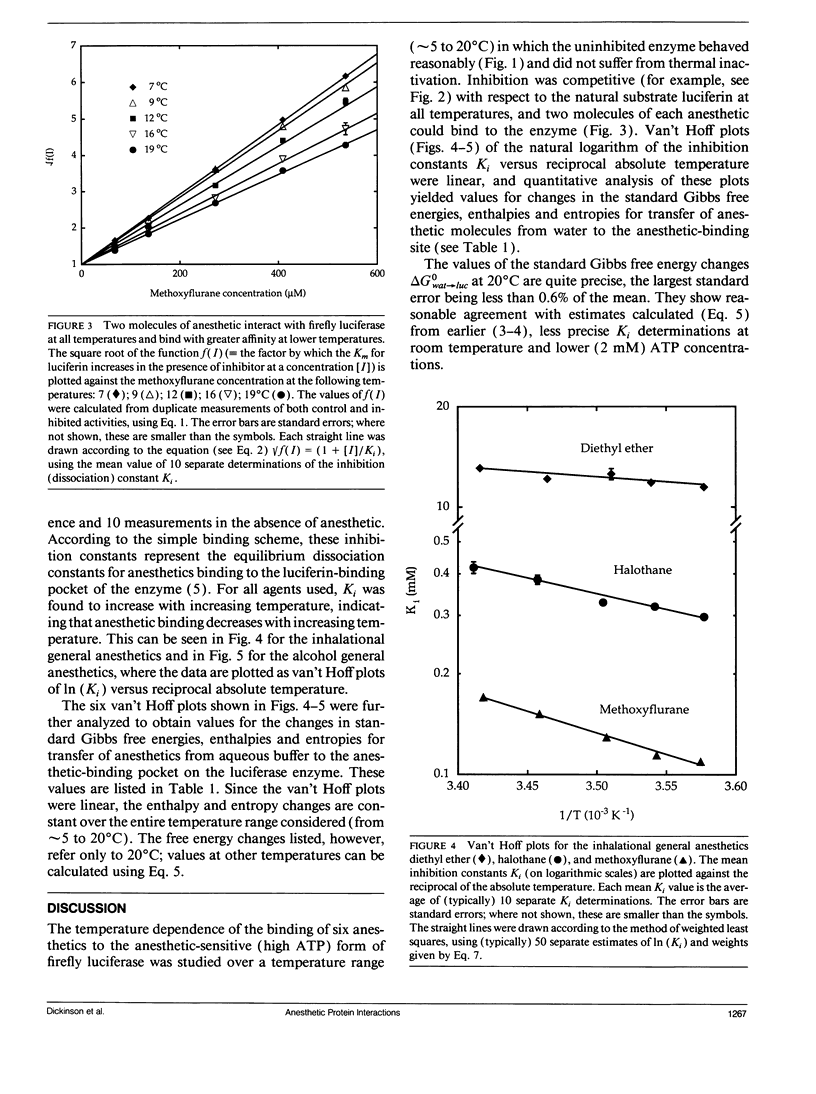
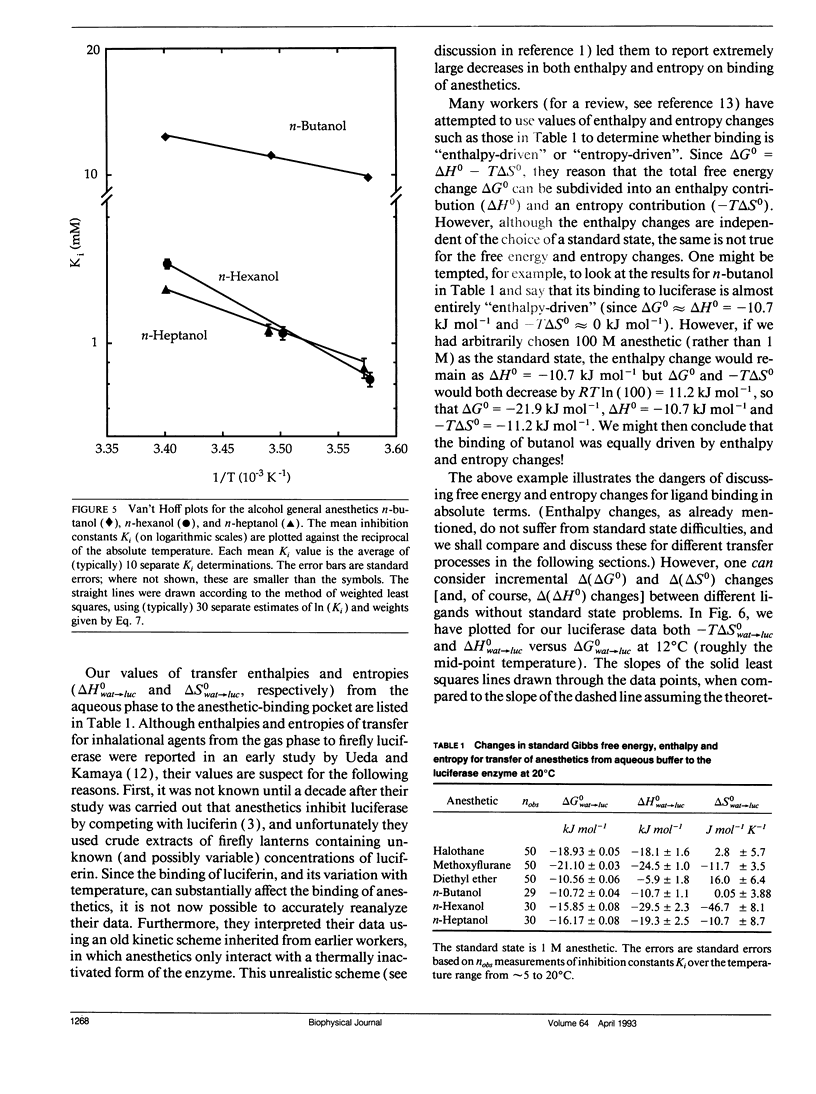
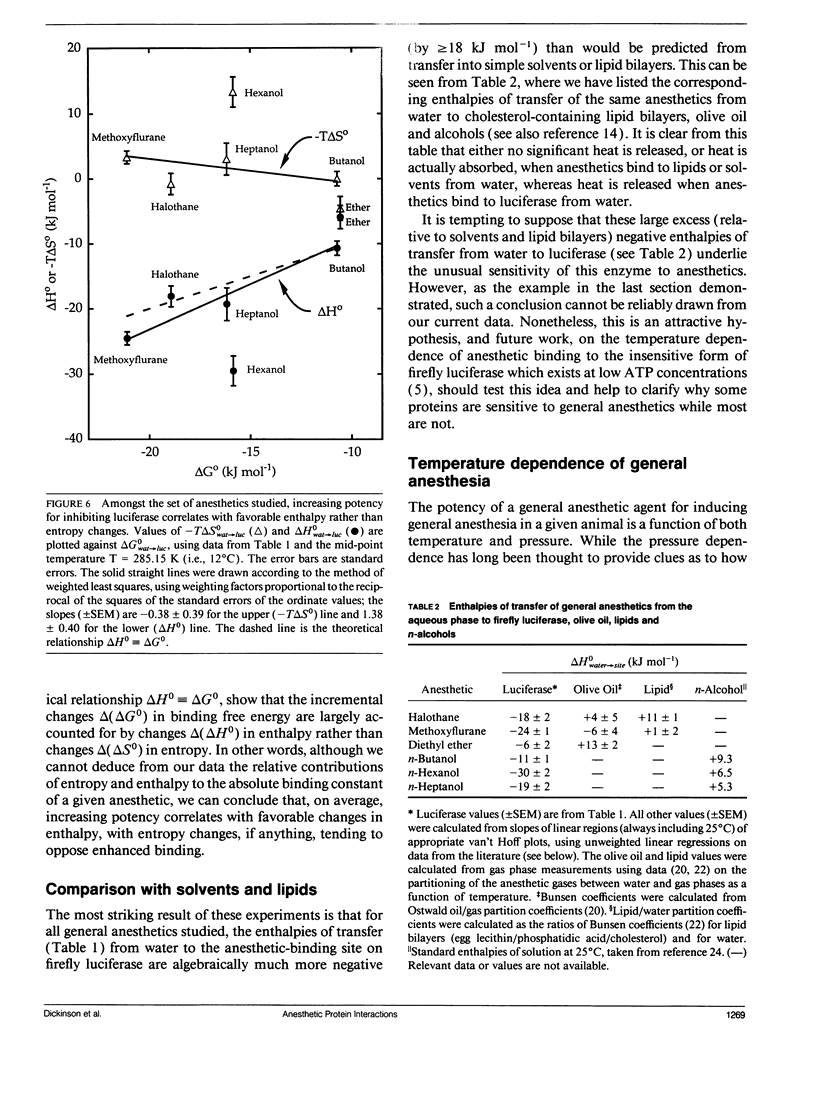
Firefly luciferase is a soluble enzyme which is unusually sensitive to general anesthetics. The inhibition of the highly purified enzyme by three inhalational and three alcohol general anesthetics has been studied as a function of temperature, in the range from 5 to 20 degrees C. Inhibition constants Ki were determined at different temperatures, and van't Hoff plots of ln (Ki) versus reciprocal absolute temperature were found to be linear for all agents. Analysis of these plots gave values for the standard Gibbs free energy, enthalpy and entropy changes for transferring each anesthetic from water to the anesthetic-binding pocket on the protein. The most striking finding was that the enthalpy changes were much more negative for anesthetics binding to the protein than for binding to lipids or simple solvents. Furthermore, amongst the set of anesthetics studied, it was found that increasing potency correlated with favorable enthalpy rather than entropy changes. We discuss our results with respect to the molecular mechanisms underlying general anesthesia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branchini B. R., Marschner T. M., Montemurro A. M. A convenient affinity chromatography-based purification of firefly luciferase. Anal Biochem. 1980 May 15;104(2):386–396. doi: 10.1016/0003-2697(80)90089-5. [DOI] [PubMed] [Google Scholar]
- CHERKIN A., CATCHPOOL J. F. TEMPERATURE DEPENDENCE OF ANESTHESIA IN GOLDFISH. Science. 1964 Jun 19;144(3625):1460–1462. doi: 10.1126/science.144.3625.1460. [DOI] [PubMed] [Google Scholar]
- Curry S., Moss G. W., Dickinson R., Lieb W. R., Franks N. P. Probing the molecular dimensions of general anaesthetic target sites in tadpoles (Xenopus laevis) and model systems using cycloalcohols. Br J Pharmacol. 1991 Jan;102(1):167–173. doi: 10.1111/j.1476-5381.1991.tb12148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E. I., 2nd, Saidman L. J., Brandstater B. Temperature dependence of halothane and cyclopropane anesthesia in dogs: correlation with some theories of anesthetic action. Anesthesiology. 1965 Nov-Dec;26(6):764–770. doi: 10.1097/00000542-196511000-00011. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Effects of physiologically relevant pressures of helium on the structure of cholesterol-containing lipid bilayers. A neutron diffraction study. Biophys J. 1991 Aug;60(2):498–501. doi: 10.1016/S0006-3495(91)82076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular mechanisms of general anaesthesia. Nature. 1982 Dec 9;300(5892):487–493. doi: 10.1038/300487a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991 Oct 18;254(5030):427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Lever M. J., Miller K. W., Paton W. D., Smith E. B. Pressure reversal of anaesthesia. Nature. 1971 Jun 11;231(5302):368–371. doi: 10.1038/231368a0. [DOI] [PubMed] [Google Scholar]
- Miller K. W. The nature of the site of general anesthesia. Int Rev Neurobiol. 1985;27:1–61. doi: 10.1016/s0074-7742(08)60555-3. [DOI] [PubMed] [Google Scholar]
- Moss G. W., Curry S., Franks N. P., Lieb W. R. Mapping the polarity profiles of general anesthetic target sites using n-alkane-(alpha, omega)-diols. Biochemistry. 1991 Oct 29;30(43):10551–10557. doi: 10.1021/bi00107a026. [DOI] [PubMed] [Google Scholar]
- Moss G. W., Franks N. P., Lieb W. R. Modulation of the general anesthetic sensitivity of a protein: a transition between two forms of firefly luciferase. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):134–138. doi: 10.1073/pnas.88.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss G. W., Lieb W. R., Franks N. P. Anesthetic inhibition of firefly luciferase, a protein model for general anesthesia, does not exhibit pressure reversal. Biophys J. 1991 Dec;60(6):1309–1314. doi: 10.1016/S0006-3495(91)82168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa R. B., Porreca F. Thermodynamic analysis of the drug-receptor interaction. Life Sci. 1989;44(4):245–258. doi: 10.1016/0024-3205(89)90182-3. [DOI] [PubMed] [Google Scholar]
- Regan M. J., Eger E. I., 2nd Effect of hypothermia in dogs on anesthetizing and apneic doses of inhalation agents. Determination of the anesthetic index (Apnea/MAC). Anesthesiology. 1967 Jul-Aug;28(4):689–700. doi: 10.1097/00000542-196707000-00015. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Bennett P. B., Shrivastav B. B. Interaction of halothane with lipid bilayers. Mol Pharmacol. 1979 Jul;16(1):163–170. [PubMed] [Google Scholar]
- Smith R. A., Porter E. G., Miller K. W. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta. 1981 Jul 20;645(2):327–338. doi: 10.1016/0005-2736(81)90204-2. [DOI] [PubMed] [Google Scholar]
- Steffey E. P., Eger E. I., 2nd Hyperthermia and halothane MAC in the dog. Anesthesiology. 1974 Oct;41(4):392–396. doi: 10.1097/00000542-197410000-00017. [DOI] [PubMed] [Google Scholar]
- Ueda I., Kamaya H. Kinetic and thermodynamic aspects of the mechanism of general anesthesia in a model system of firefly luminescence in vitro. Anesthesiology. 1973 May;38(5):425–436. doi: 10.1097/00000542-197305000-00002. [DOI] [PubMed] [Google Scholar]


